Press release
English
Swedish
![]()
2 October 2006
The Nobel Assembly at Karolinska Institutet has today decided to award
The Nobel Prize in Physiology or Medicine for 2006 jointly to
Andrew Z. Fire and Craig C. Mello
for their discovery of “RNA interference – gene silencing by double-stranded RNA“
Summary
This year’s Nobel Laureates have discovered a fundamental mechanism for controlling the flow of genetic information. Our genome operates by sending instructions for the manufacture of proteins from DNA in the nucleus of the cell to the protein synthesizing machinery in the cytoplasm. These instructions are conveyed by messenger RNA (mRNA). In 1998, the American scientists Andrew Fire and Craig Mello published their discovery of a mechanism that can degrade mRNA from a specific gene. This mechanism, RNA interference, is activated when RNA molecules occur as double-stranded pairs in the cell. Double-stranded RNA activates biochemical machinery which degrades those mRNA molecules that carry a genetic code identical to that of the double-stranded RNA. When such mRNA molecules disappear, the corresponding gene is silenced and no protein of the encoded type is made.
RNA interference occurs in plants, animals, and humans. It is of great importance for the regulation of gene expression, participates in defense against viral infections, and keeps jumping genes under control. RNA interference is already being widely used in basic science as a method to study the function of genes and it may lead to novel therapies in the future.
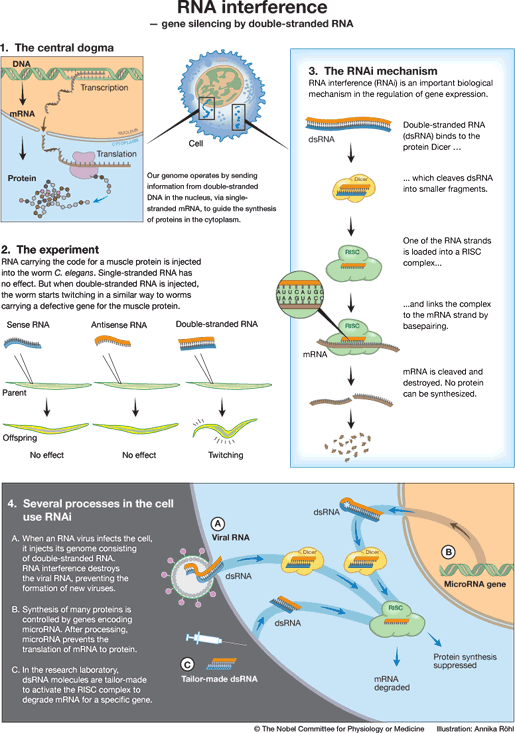
The flow of information in the cell: from DNA via mRNA to protein
The genetic code in DNA determines how proteins are built. The instructions contained in the DNA are copied to mRNA and subsequently used to synthesize proteins (Fig 1). This flow of genetic information from DNA via mRNA to protein has been termed the central dogma of molecular biology by the British Nobel Laureate Francis Crick. Proteins are involved in all processes of life, for instance as enzymes digesting our food, receptors receiving signals in the brain, and as antibodies defending us against bacteria.
Our genome consists of approximately 30,000 genes. However, only a fraction of them are used in each cell. Which genes are expressed (i.e. govern the synthesis of new proteins) is controlled by the machinery that copies DNA to mRNA in a process called transcription. It, in turn, can be modulated by various factors. The fundamental principles for the regulation of gene expression were identified more than 40 years ago by the French Nobel Laureates François Jacob and Jacques Monod. Today, we know that similar principles operate throughout evolution, from bacteria to humans. They also form the basis for gene technology, in which a DNA sequence is introduced into a cell to produce new protein.
Around 1990, molecular biologists obtained a number of unexpected results that were difficult to explain. The most striking effects were observed by plant biologists who were trying to increase the colour intensity of the petals in petunias by introducing a gene inducing the formation of red pigment in the flowers. But instead of intensifying the colour, this treatment led to a complete loss of colour and the petals turned white! The mechanism causing these effects remained enigmatic until Fire and Mello made the discovery for which they receive this year’s Nobel Prize.
The discovery of RNA interference
Andrew Fire and Craig Mello were investigating how gene expression is regulated in the nematode worm Caenorhabditis elegans (Fig. 2). Injecting mRNA molecules encoding a muscle protein led to no changes in the behaviour of the worms. The genetic code in mRNA is described as being the ‘sense’ sequence, and injecting ‘antisense’ RNA, which can pair with the mRNA, also had no effect. But when Fire and Mello injected sense and antisense RNA together, they observed that the worms displayed peculiar, twitching movements. Similar movements were seen in worms that completely lacked a functioning gene for the muscle protein. What had happened?
When sense and antisense RNA molecules meet, they bind to each other and form double-stranded RNA. Could it be that such a double-stranded RNA molecule silences the gene carrying the same code as this particular RNA? Fire and Mello tested this hypothesis by injecting double-stranded RNA molecules containing the genetic codes for several other worm proteins. In every experiment, injection of double-stranded RNA carrying a genetic code led to silencing of the gene containing that particular code. The protein encoded by that gene was no longer formed.
After a series of simple but elegant experiments, Fire and Mello deduced that double-stranded RNA can silence genes, that this RNA interference is specific for the gene whose code matches that of the injected RNA molecule, and that RNA interference can spread between cells and even be inherited. It was enough to inject tiny amounts of double-stranded RNA to achieve an effect, and Fire and Mello therefore proposed that RNA interference (now commonly abbreviated to RNAi) is a catalytic process.
Fire and Mello published their findings in the journal Nature on February 19, 1998. Their discovery clarified many confusing and contradictory experimental observations and revealed a natural mechanism for controlling the flow of genetic information. This heralded the start of a new research field.
The RNA interference machinery is unraveled
The components of the RNAi machinery were identified during the following years (Fig 3). Double-stranded RNA binds to a protein complex, Dicer, which cleaves it into fragments. Another protein complex, RISC, binds these fragments. One of the RNA strands is eliminated but the other remains bound to the RISC complex and serves as a probe to detect mRNA molecules. When an mRNA molecule can pair with the RNA fragment on RISC, it is bound to the RISC complex, cleaved and degraded. The gene served by this particular mRNA has been silenced.
RNA interference – a defense against viruses and jumping genes
RNA interference is important in the defense against viruses, particularly in lower organisms. Many viruses have a genetic code that contains double-stranded RNA. When such a virus infects a cell, it injects its RNA molecule, which immediately binds to Dicer (Fig 4A). The RISC complex is activated, viral RNA is degraded, and the cell survives the infection. In addition to this defense, higher organisms such as man have developed an efficient immune defense involving antibodies, killer cells, and interferons.
Jumping genes, also known as transposons, are DNA sequences that can move around in the genome. They are present in all organisms and can cause damage if they end up in the wrong place. Many transposons operate by copying their DNA to RNA, which is then reverse-transcribed back to DNA and inserted at another site in the genome. Part of this RNA molecule is often double-stranded and can be targeted by RNA interference. In this way, RNA interference protects the genome against transposons.
RNA interference regulates gene expression
RNA interference is used to regulate gene expression in the cells of humans as well as worms (Fig 4B). Hundreds of genes in our genome encode small RNA molecules called microRNAs. They contain pieces of the code of other genes. Such a microRNA molecule can form a double-stranded structure and activate the RNA interference machinery to block protein synthesis. The expression of that particular gene is silenced. We now understand that genetic regulation by microRNAs plays an important role in the development of the organism and the control of cellular functions.
New opportunities in biomedical research, gene technology and health care
RNA interference opens up exciting possibilities for use in gene technology. Double-stranded RNA molecules have been designed to activate the silencing of specific genes in humans, animals or plants (Fig 4C). Such silencing RNA molecules are introduced into the cell and activate the RNA interference machinery to break down mRNA with an identical code.
This method has already become an important research tool in biology and biomedicine. In the future, it is hoped that it will be used in many disciplines including clinical medicine and agriculture. Several recent publications show successful gene silencing in human cells and experimental animals. For instance, a gene causing high blood cholesterol levels was recently shown to be silenced by treating animals with silencing RNA. Plans are underway to develop silencing RNA as a treatment for virus infections, cardiovascular diseases, cancer, endocrine disorders and several other conditions.
Reference:
Fire A., Xu S.Q., Montgomery M.K., Kostas S.A., Driver S.E., Mello C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998; 391:806-811.
Andrew Z. Fire, born 1959, US citizen, PhD in Biology 1983, Massachusetts Institute of Technology, Cambridge, MA, USA. Professor of Pathology and Genetics, Stanford University School of Medicine, Stanford, CA, USA.
Craig C. Mello, born 1960, US citizen, PhD in Biology 1990, Harvard University, Boston, MA, USA. Professor of Molecular Medicine and Howard Hughes Medical Institute Investigator, Program in Molecular Medicine, University of Massachusetts Medical School, Worcester, MA, USA.
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 14 laureates' work and discoveries range from quantum tunnelling to promoting democratic rights.
See them all presented here.
