Protein synthesis
|
How do newly synthesized proteins find their correct destinations within a cell, and how are they able to pass across the tightly sealed intracellular membranes? These were the central questions that Günter Blobel began to address in the late 1960s. He started by analyzing how newly synthesized secretory proteins are first targeted to and then translocated across the membrane of the endoplasmic reticulum (ER). These two steps are prerequisites for secretion of proteins out of the cell. |
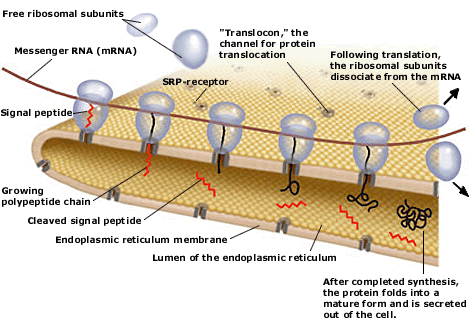 |
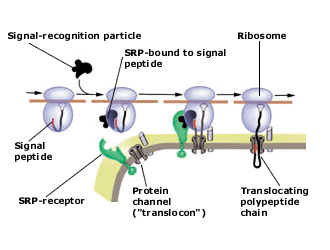

Present view of protein translocation across the ER membrane. The signal peptide, emerging from the ribosome, binds to the signal-recognition particle (SRP). The SRP-ribosome complex then docks to the SRP-receptor and channel (“translocon”).
SRP dissociates from the receptor and the nascent polypeptide chain is translocated through the channel into the ER lumen. The signal peptide is finally cleaved and the protein is secreted out of the cell.
the protein translocating channel (the “translocon”).
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 14 laureates' work and discoveries range from quantum tunnelling to promoting democratic rights.
See them all presented here.
