Speed read: Creating supply on demand
The 1984 Nobel Prize in Physiology or Medicine celebrated the important contribution of theory and practice in shaping our understanding of the body’s immune system. The hypotheses formulated by Nils Jerne presented a clearer image of the way in which a diverse range of antibodies can be engaged to fight an invader. Georges Köhler and César Milstein constructed perpetual antibody-production lines that have become an essential laboratory tool for researchers worldwide.
Jerne’s first major theory, published in 1955, refuted the general opinion that the immune system custom designs new antibodies when it encounters unfamiliar molecules, or antigens, on an intruder. The body has already created its full repertoire of antibodies, proposed Jerne, and it selects the correct one for the task – a hypothesis later refined by MacFarlane Burnet, which stated that each individual white blood cell produces only one specific antibody. Jerne’s network theory in 1975 described how the immune response is exquisitely controlled, and was built on his premise that antibodies can themselves act as antigens. With the various sets of antibodies stimulating or suppressing the production of each other, he visualized the immune system as a self-regulating network that can switch itself on and off in response to a foreign invasion.
Köhler and Milstein were independently trying to test theories about antibody production in the laboratory, and to do so they both sought ways of creating long-living cell lines that could generate large amounts of a particular antibody. Milstein had developed cancerous forms of antibody-producing cells that grew and multiplied forever, but which churned out antibodies of unknown specificity; while Köhler had tweaked normal antibody-producing cells to produce specific antibodies, but they survived for a few days only in culture. Combining forces, the neat trick they came up with was to fuse a normal antibody-producing cell with a tumour cell, forming a hybrid that was both immortal and could create a specific antibody. Köhler and Milstein’s technique for creating any single predetermined type of so-called monoclonal antibody on demand has led to many medicine and biomedical applications, from creating more reliable probes for blood and tissue typing tests, to designing completely new therapeutic strategies for diseases such as cancer.
This Speed read is an element of the multimedia production “Immune Responses”. “Immune Responses” is a part of the AstraZeneca Nobel Medicine Initiative.
Niels K. Jerne – Other resources
Links to other sites
Interview with Niels Jerne from BBC Archive: Discovering how our immune systems protect us
Niels K. Jerne – Facts
Georges J.F. Köhler – Facts
Press release

NOBELFÖRSAMLINGEN KAROLINSKA INSTITUTET
THE NOBEL ASSEMBLY AT THE KAROLINSKA INSTITUTE
The Nobel Assembly of Karolinska Institutet has today decided to award the Nobel Prize in Physiology or Medicine for 1984 jointly to
Niels K. Jerne, Georges J.F. Köhler and César Milstein
for theories concerning “the specificity in development and control of the immune system” and the discovery of “the principle for production of monoclonal antibodies”.
Summary
Niels K. Jerne is the great theoretician in immunology. In three main theories he has in a visionary way elucidated essential questions concerning specificity, development and regulation of the immune response. The natural-selection theory regarding antibody formation breaks with old views on the immune response and is a starting point of modern cellular immunology. His second theory explains how the cells of the immune system which mature in the thymus gland develop under the influence of the transplantation antigens of the host. The third, and most important theory, predicts how the immune response is regulated by a complicated network consisting of antibodies and anti-anti-bodies. The principles of the network theory are beginning to be exploited in prevention, diagnosis and treatment of disease.
The hybridoma technique for the production of monoclonal antibodies represents one of the most important methodological advances in biomedicine during the 1970s. An antibody producing cell and all its daughter cells produce an identical antibody molecule (monoclonal antibody). Since long scientists nourished the hope that it would become possible to produce monoclonal antibodies with predetermined specificities. This dream became a reality in 1975 when Georges J.F. Köhler and César Milstein described the hybridoma technique for production of monoclonal antibodies. They immortalized antibody producing cells by fusing them with tumour cells. The method allows unlimited production of monoclonal antibodies with predetermined specificity. Monoclonal antibodies has opened up completely new fields for theoretical and applied biomedical research and allows precise diagnosis and also treatment of disease.
The most important task for the immune system is to defend the body against bacteria, virus and other microorganisms. The specific defense is exerted by a subgroup of white blood cells (lymphocytes). The immune system needs to recognize and react specifically with a large number of foreign substances (antigens). How the lymphocytes develop these vital properties and how they build up the highly specialized recognition system of the immune apparatus has long been an area of intensive research.
Niels K. Jerne is the leading theoretician in immunology during the last 30 years. In three main theories he has elucidated central issues concerning specificity, development and regulation of the immune system in a comprehensive and convincing way. By his theories Jerne has outlined the development of modern immunology.
Theory 1: Specificity is predetermined
In his Natural-Selection Theory of Antibody Formation from 1955 Jerne explains the development of a specific antibody response in the following way. Each individual has a large number of natural antibodies with specificities for all antigens towards which the individual can respond. These antibodies develop already during fetal life in the absence of external antigens. The foreign antigen then selects the antibody molecule which has the best fit. The antigen-antibody binding stimulates the production of this particular antibody specificity.
Jerne’s natural-selection theory contrasted to the dogmatic views of the antibody response as formulated in the instruction theories which were prevailing at that time. According to these theories the antigen serves a template for the production of antibodies.
In Jerne’s natural selection theory it is implied that the generation of the enormous number of antibody specificities is independent of exogenous antigens. This view on the nature of the immune system constitutes the basis for modern immunology.
Theory 2: Reactivity against self-antigens creates diversity
The natural-selection theory is mainly concerned with the maturation of the immune system after it has acquired the ability to react with antigen. In the second theory on the Somatic Generation of Immune Recognition set forth in 1971 Jerne explains how the immune system develops from stem cells to mature lymphocytes which can react with antigen. He presupposes that every individual possesses all genes needed for the production of antibodies, and antibody-like molecules, which can bind all strong transplantation antigens of the species. Jerne suggests that lymphocytes mature in the thymus gland and in other lymphoid organs where they are exposed to the transplantation antigens of the individual. Cells which recognize the antigens are stimulated and enter cell division. As mutations accumulate in rapidly dividing cells new immunological specificities may develop. At the same time the specificities of the lymphocytes for self transplantation antigens are weakened. The mature lymphocytes will recognize foreign antigen associated with transplantation antigens. The theory explains how the immune system normally matures through the influence of self antigens. It also offers an explanation for the regulation of immunological specificity by genes belonging to the transplantation system.
Theory 3. Antibodies, anti-anti-bodies …
In his third main theory, the Network Theory from 1974, Jerne explains how the specific immune response is regulated. The theory has greatly stimulated research and led to new insights into the immune system. Recently its principles have been applied to diagnosis and treatment of disease.
A basis for the network theory was the observation that antibodies can elicit anti-antibodies directed against antigen binding structures on the first antibody (Figure 1). Moreover, anti-antibodies can stimulate the production of still another generation of antibodies, anti-anti-antibodies. Essentially, this antibody cascade is endless successively adding new specific properties to the immune system. The various antibody generations either stimulate or suppress the production of one another. Under normal conditions the network is balanced. When an antigen is introduced the equilibrium is disturbed. The immune system tries to restore balance which leads to an immune response against the antigen.
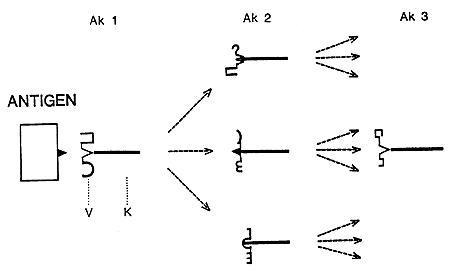 |
Figure 1. The Network Theory. Antibody 1 (Ak-1) has a structure in its variable (V) region which can bind the antigen. The V-region of Ak-1 contains unique structures which stimulate the production of various anti-antibodies (Ak-2). Some Ak-2 express V-region structures which mimic the antigen and which therefore can stimulate Ak-1 production.
Each antibody generation induces the production of still another and larger set of anti-antibodies in a cascade-like manner. The various sets of antibodies stimulate or suppress the production of each other in a complex network. Under normal conditions the network is balanced. However, the equilibrium is disturbed when an antigen is introduced and binds to Ak-1. The immune system attempts to restore the balance, i.e. it leads to an immune response.
Some examples where the network theory has been applied to experimental and clinical medicine are given in the following.
1. Infectious diseases. Anti-antibodies have been used in animals as a kind of vaccine against parasitic infections (trypanosomiasis), urinary tract infections, hepatitis and other infectious diseases.
2. Allergy. Anti-pollen antibodies may elicit allergic symptoms when an allergic person is exposed to pollen. The production of anti-pollen antibodies has been prevented in animals by anti-antibodies.
3. Autoimmune disease. Autoimmune disease may be caused by antibodies directed against the body’s own tissues. Experimental autoimmune disease has been successfully treated with anti-antibodies.
4. Transplantation. Anti-antiimmunity may be important in organ transplantation by contributing to immunological tolerance against antigen on the foreign graft.
5. Endocrinology. Anti-antibodies against hormones and hormone receptors may prevent binding of the hormone to the receptors. This has been described for insulin and its receptor.
6. Tumours. Anti-antibodies have been attempted as treatment of certain tumours of the human immune system.
Hybridoma – a technique for eternal production of monoclonal antibodies in cell cultures
Besides gene technology, which has already been honoured by several Nobel Prizes, the hybridoma technique represents the most important methodological advance within the field of biomedicine during the 1970s. The development of this technique is based on several observations concerning basic biological phenomena.
There are cells in the body – immune lymphocytes – which can produce millions of different antibodies. However, each single cell can only produce antibodies with a certain predetermined specificity. A prerequisite for the formation of a multitude of antibodies is, therefore, the existence of an excess of lymphocytes. If the body is exposed to a certain foreign antigen there may be stimulation of a lymphocyte which fortuitously has been endowed with the capacity to identify this particular antigen. This lymphocyte then starts to divide and forms a clone of cells which produces identical – monoclonal – antibodies.
The development of a clone of cells in connection with a normal immune response occurs under carefully controlled conditions. In rare cases, however, the body loses control over a clone of antibody producing cells. This may lead to formation of a special type of tumour (myeloma). Myeloma cells usually retain their capacity to produce a certain antibody, but because of the accidental emergence of the tumour one normally does not know with which antigen this antibody reacts.
White blood cells responsible for producing antibodies are highly specialized cells. As a consequence they lack capacity to survive for a longer time if they are removed from the body and incubated in a tissue culture medium. In contrast, myeloma cells can occasionally be cultivated continuously. Since long, biomedical research workers have nourished the dream to be able to propagate clones of cells which produce antibodies with predetermined specificity. This dream materialized when Georges J.F. Köhler and César Milstein in 1975 introduced the so-called hybridoma technology for production of monclonal antibodies. The principle features of the hybridoma technology is as follows (Figure 2).
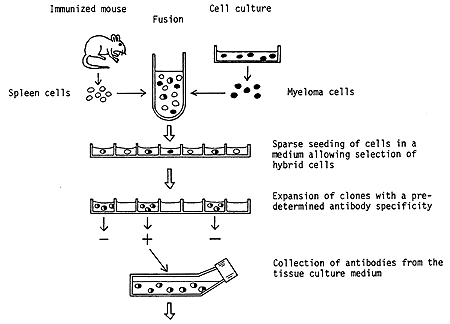
Figure 2. Principle steps in the production of a hybridoma. Spleen cells are prepared from animals, usually mice, which have been immunized with a selected antigen. These cells are then fused with myeloma cells maintained in culture in the laboratory. The product of this fusion is referred to as a hybridoma. Surprisingly, a hybrid of two cells can survive and also continue to divide. In this particular hybrid the myeloma cells contribute the capacity for survival, whereas the spleen cells direct the synthesis of antibodies with the preselected specificity. By special arrangements it is possible to achieve a multiplication of hybridoma cells but not of isolated myeloma cells. The hybrids obtained are propagated in a highly diluted state so that colonies deriving from single hybrid cells can be isolated. By use of a sensitive method the clones which produce the specific antibodies are identified. A particular hybridoma can then be used for future, unlimited production of a highly specific antibody.
The availability of monoclonal antibodies has opened completely new possibilities for basic as well as applied biomedical research. The following examples of the use of monoclonal antibodies can be given.
1. Detailed studies of the distribution of different functions in different parts of antigen molecules. These studies may concern building elements of infectious agents; cell products such as enzymes and hormones; surface structures of cells etc. The mapping of variations in the surface components of influenza virus which explain the occurrence of repeated infections is one example.
2. High degree purification of substances, e.g. interferon, by taking advantage of the unique capacity displayed by a particular monoclonal antibody to bind to a certain antigen. In this case one uses a technique referred to as affinity chromatography.
3. Diagnostic characterization of diseases by identification of special structures on the surface or on the inside of cells. Hereby it is possible to distinguish between different forms of tumours and follow the development of tumours. Furthermore, it is possible to distinguish between different kinds of normal white blood cells. This is of importance for the characterization of certain immune deficiency conditions as seen e.g. in connection with the disease AIDS (acquired immune deficiency syndrome).
Diseases caused by infectious agents can also be diagnosed by use of monoclonal antibodies. Thus, virus infected cells and bacteria or parasites inside or outside cells can be identified with a unique degree of specificity.
4. Treatment of diseases. Monoclonal antibodies against specialized white blood cells have been used with some success in connection with transplantation. There may also be possibilities to use monoclonal antibodies for treatment of tumours.
References
C. Milstein: Monoclonal Antibodies. Scientific American 1980, vol. 243, pp. 56-65.
Hybridomas: The Making of a Revolution. Science 1982, vol. 215, pp. 1073-1075.
Odödliga hybridceller – fabriker för tillverkning av monoklonala antikroppar. En av vår tids mest lovande medicinska upptäckter. Läkartidningen 1982, vol. 79, pp. 3545-3546.
L. Å. Hanson H. Wigzell: Immunologi, Del I. Teori. Almqvist & Wiksell, Stockholm, 1983.
M. Harboe & J. B. Natvig: Medisinsk Immunologi. Grøndahl & Søn Trykkeri A.s., Oslo, 1977.
César Milstein – Facts
Award ceremony speech
Presentation Speech by Professor Hans Wigzell of the Karolinska Institute
Translation from the Swedish text
Your Majesties, Your Royal Highnesses, Ladies and Gentlemen,
It is typical for the human mind that little thought goes to the functions of our body when we are healthy, yet acute interest frequently develops in times of disease. The immune system is a somewhat anonymous, talented and well-trained cellular society within ourselves which must function properly to maintain our health. The immune defence has the inherent capacity to rapidly recognize foreign material and can subsequently remember this contact for decades, thus creating the basis for vaccination. Through a clever usage of genetic material and large numbers of cells, the immune system within a single human being is able to produce defence molecules, antibodies, in billions of different shapes. The Nobel prize winners in physiology or medicine this year have all worked with the capacity of the immune system to produce specific antibodies.
Niels Jerne is the great theoretician in modern immunology. He entered the immunological arena comparatively late in his life and was 44 years old when in 1955 he published his first important theory of the construction of the immune system. Jerne proposed that the well-known capacity of the immune defence to recognize myriades of foreign molecules was something predetermined, already existing in the body when the very first contact with a foreign structure was made. What then happened was merely a selection amongst the naturally occurring antibody population resulting in an increase in production of exactly those antibodies which happened to have a good fit for the structure. Jerne’s theory stood in great contrast to prevailing theories at that time, but it was rapidly confirmed and extended. We now know that Darwin’s laws about natural selection indeed apply to the cells of the immune system: Those cells which happen to have received the property to produce a wanted antibody type will upon vaccination be rewarded with regard to proliferative capacity and survival.
Jerne took another known feature of the immune defence as the starting point for his next important theory, in 1971. The immune system always expresses a very strong defence of the private and unique features of the tissues within one individual. This behaviour creates great problems whenever attempts are made to transplant tissue from one individual to another. Jerne assumed that the molecules in the tissues that cause these reactions, the so-called transplantation antigens, must have their normal functions within the body of the individual. He proposed that one function of these molecules could be to serve as a specific driving force for the cells of the immune system, thus creating a large number of cells from which cells especially suitable to defend the tissues of the host would become selected. Special organs, such as the thymus, were assumed to serve as a combination of greenhouse and university for these cells. In this theory Jerne did predict to a great extent how the specificity of the cellmediated immunity is generated.
In the third great theory, in 1974, Niels Jerne introduced us to the mirror halls of immunology. The immune system is visualized somewhat like a gigantic computer where constant communication and regulation takes place in between the different components, the cells. The number of cells in such a network system in an adult human being exceeds 1012 (one million millions); and the system has through its capacity to produce billions of different forms of antibodies an enormous inbuilt richness with regard to structural variations. Jerne proposed that this should allow the creation of multiple complementary situations where certain antibodies would have select capacity to combine with their mirror images. Some antibodies would according to the theory then even mimic foreign molecules against which other antibodies would normally be produced during immunization. Jerne postulated that pairs of antibodies and their mirror images would spontaneously be produced during the development of the immune system, thus creating the possibilities for communication networks and regulatory equlibria. During immunization the foreign molecules would enter the mirror halls of immunology where the different pairs of antibodies and cells would perform their interdependent piruettes and separate the partners chasing away the mirror images. This change in equilibrium would then serve as a driving force, resulting in immunity. It is now well documented that network forces of the fascinating type that Jerne predicted do indeed exist inbuilt in our own immune systems. The theory also predicted the almost mind-boggling possibility that antibodies of the mirror image-type could replace the foreign material completely when inducing immunity. This is now a proven reality. Thus, it is for instance possible to induce a long lasting immunity against hepatitis virus by immunization with mirror image antibodies to the antibodies against hepatitis virus without ever using the virus in the vaccination.
In conclusion, Niels Jerne has via his visionary theories enabled modern immunology to make major leaps of progress. Several concepts in immunology now considered as self-evident have their roots in some of his pioneering thoughts.
In order to fully understand the importance of Georges Köhler’s and César Milstein’s discoveries we should first take some steps back. Sera from intentionally immunized animals or humans constitute very important tools in the hospitals as well as in research laboratories. They are used to diagnose infectious diseases, as well as to determine the concentration of a particular hormone in a sample. But every one of these immune sera contains a unique mixture of antibodies produced by a large number of different cells and their progeny and the various antibodies react in a similar yet distinctly different manner. Thus, each immune serum has to be tested to determine the special features of that particular serum with regard to its ability to distinguish between two related hormones, different bacteria, etc. Regardless of whether the immune serum is close to being perfect or not, it will always be used up, and it is then necessary to start again trying to produce a similar kind of serum. International standardizations of tests using immune sera have thus been greatly hampered.
The discovery and development of principles for production of the so-called monoclonal antibodies by the hybridoma technique by Georges Köhler and César Milstein have largely solved all the above major problems. And the story of the discovery of the technique also contains the moral of a saga, where the evil is put into the service of the good. How did this discovery take place? César Milstein is a highly prominent biochemist working for a long time in Cambridge in England. A major interest in his research has been to explore various facets of antibody production. Milstein used tumor cells which had arisen in cells of a type that normally produce antibodies. Such tumors also produce proteins which in all respects look like antibodies, although it is difficult to find suitable foreign structures to which they can bind. Milstein wanted amongst other things to study what would happen if two different tumor lines were allowed to fuse, e.g. what would happen to the production of the antibody-like proteins if for instance the tumor cells came from different species? Milstein constructed tumor cell lines allowing only hybrid cells between the two tumor cells to grow in certain defined tissue culture solutions. The systems worked and the hybrid cells produced large quantities of the antibody-like proteins, some of which at the molecular level could be shown to be hybrid molecules as well.
At the same time the young researcher Georges Köhler struggled in Basel in Switzerland to study normal antibody-producing cells in tissue culture. His research was in part frustrating as he could only get very few cells to survive for short periods of time. Köhler knew of the important studies of Milstein, and it seemed logical to see if normal antibody-forming cells could be fused with tumor cells to produce long-lived hybrid cell lines. If this was indeed possible the experiments of Milstein would indicate that they should then continue to produce their antibodies. At the same time the normally evil feature of tumor cells, the capacity to proliferate for ever, would now be turned into a very beneficial feature. Köhler went to Milstein’s laboratory and together they wrestled with the problems and managed to solve them in a hectic two year period, 1975-1976. By that time they had succeeded to develop a technique allowing them at will to fish up exactly those rare antibody-producing cells that they wanted from a sea of cells. These cells were fused with tumor cells creating hybrid cells with eternal life and capacity to produce the very same antibody in high quantity. Köhler and Milstein called these hybrid cells hybridomas, and as all cells in a given hybridoma come from one single hybrid cell, the antibodies made are monoclonal.
Köhler’s, and Milstein’s development of the hybridoma technique for production of monoclonal antibodies have in less than a decade revolutionized the use of antibodies in health care and research. Rare antibodies with a tailor-made-like fit for a given structure can now be made in large quantities. The hybridoma cells can be stored in tissue banks and the very same monoclonal antibody can be used all over the world with a guarantee for eternal supply. The precision in diagnosis is greatly improved, and entirely new possibilities for therapy have been opened up via the hybridoma technique. Rare molecules present in trace amounts in complex solution can now be purified in an efficient manner using monoclonal antibodies. In all, it is therefore correct to describe the hybridoma technique discovered by Georges Köhler and César Milstein as one of the major methodological advances in medicine during this century.
Dr. Jerne, Dr. Köhler and Dr. Milstein,
On behalf of the Nobel Assembly of the Karolinska Institute I would like to congratulate you on your outstanding accomplishments and ask you to receive the Nobel Prize in Physiology or Medicine from the hands of His Majesty the King.
The Nobel Prize in Physiology or Medicine 1984
Niels K. Jerne – Biographical

Niels K. Jerne, born 23rd December 1911, London
My parents, Hans Jessen Jerne and Else Marie Lindberg, and their ancestors (back to the seventeenth century and earlier) all lived on the island Fanø and in a small adjacent area of western Jutland in Denmark. My family moved to London in 1910, and then to Holland during the first world war. I received my Baccalaureate in Rotterdam in 1928.
After two years of studying physics at the University of Leiden, I switched to medicine at the University of Copenhagen where I presented my thesis on the avidity of antibodies in 1951.
My wife Alexandra and I married in 1964, and now live in our house near Avignon. Further details of my curriculum vitae:
| Research worker at the Danish State Serum Institute (1943-1956) |
| Research fellow at the California Institute of Technology, Pasadena (1954-1955) |
| Head of the Sections of Biological Standards and of Immunology at the World Health Organization, Geneva (1956-1962) |
| Professor of Biophysics at the University of Geneva (1960 – 1962) |
| Professor of Microbiology and Chairman of the Department, University of Pittsburgh (1962-1966) |
| Professor of Experimental Therapy at the Johann-Wolfgang-Goethe-Universität, Frankfurt, and Director of the Paul-Ehrlich-Institut, Frankfurt (1966-1969) |
| Director of the Basel Institute for Immunology, Basel (1969-1980) |
| Special Immunology Adviser to the Director of the Institut Pasteur, Paris (1981-1982) |
| Member emeritus and Honorary Chairman of the Advisory Board of the Basel Institute for Immunology (from 1981) |
| Member of the WHO Advisory Committee on Medical Research (1949-1968) |
| Member of the Advisory Committee on Medical Research of the Panamerican Health Organization (1963-1966) |
| Member of the Expert Advisory Panel of Immunology of the WHO since 1962 |
| Honorary Member of the Robert-Koch-Institut, Berlin (1966) |
| Foreign Honorary Member of the American Academy of Arts and Sciences (1967) |
| Member of the Royal Danish Academy of Sciences (1969) |
| Chairman, Council of the European Molecular Biology Organization (1971-1975) |
| Gairdner Foundation International Award, Toronto (1970) |
| Doctor of Science, h.c., University of Chicago (1972) |
| Honorary Member of the American Association of Immunologists (1973) |
| Foreign Associate of the National Academy of Sciences (USA) (1975) |
| Waterford Bio-Medical Science Award, La Jolla (1978) |
| Doctor of Science, h.c., Columbia University, New York (1978) |
| Foreign Member of the American Philosophical Society (1979) |
| Doctor of Science, h.c., University of Copenhagen (1979) |
| Marcel Benoist Prize, Bern (1979) |
| Fellow of the Royal Society (1980) |
| Doctor of Science, h.c., University of Basel (1981) |
| Member of the Académie des Sciences de l’Institut de France (1981) |
| Paul Ehrlich Prize, Frankfurt (1982) |
| Honorary Member of the British Society for Immunology (1983) |
| Doctor of Medicine, h.c., Erasmus University, Rotterdam (1983) |
| The work referred to in the citation for the award of the Nobel Prize is mainly included in the following papers: |
| “The natural selection theory of antibody formation” Proc. Nat. Acad. Sci. USA 41, 849-857, 1955 |
| “Immunological speculations” Ann. Rev. Microbiol. 14, 341-358, 1960 |
| “Plaque formation in agar by single antibody-producing cells” (with Albert A. Nordin), Science 140, 405, 1963 |
| “The natural selection theory of antibody formation: ten years later” in “Phage and the origins of molecular biology” Cold Spring Harbor Lab. of Quant. Biology 301-312, 1966 |
| “Antibodies and learning” in “The Nerurosciences”, The Rockefeller University Press 200-205, 1967 |
| “Waiting for the End” Cold Spring Harbor Symp. on Quant. Biology 32, 591-603, 1967 |
| “The somatic generation of immune recognition” Eur. J. Immunol. 1, 1-9, 1971 |
| “What precedes clonal selection?” in “The ontogeny of Acquired Immunity”, Ciba Foundation Symposium, Elsevier, Amsterdam 1-15, 1972 |
| “Towards a network theory of the immune system” Ann. Immunol. (Inst. Pasteur) 125C, 373-389, 1974 |
| “The immune system: a web of v-domains” Academic Press, New York, Harvey Lectures 70, 93-110, 1976 |
| “Idiotypic networks and other preconceived ideas”, Immunological Reviews 79, 5-24, 1984 |
This autobiography/biography was written at the time of the award and later published in the book series Les Prix Nobel/ Nobel Lectures/The Nobel Prizes. The information is sometimes updated with an addendum submitted by the Laureate.
Niels K. Jerne died on October 7, 1994.
César Milstein – Biographical

My father was a Jewish immigrant who settled in Argentina, and was left to his own devices at the age of 15. My mother was a teacher, herself the daughter of a poor immigrant family. For both my mother and my father, no sacrifice was too hard to make sure that their three sons (I was the middle one) would go to university. I wasn’t a particularly brilliant student, but on the other hand I was very active in Student Union affairs and in student politics. It was in this way that I met my wife, Celia. After graduation, we married, and took a full year off in a most unusual and romantic honeymoon, hitch-hiking our way through most countries in Europe, including a couple of months working in Israel kibbutzim. As we returned to Argentina, I started seriously to work towards a doctoral degree under the direction of Professor Stoppani, the Professor of Biochemistry at the Medical School. My PhD thesis work was done with no economic support. Both Celia and I worked part-time doing clinical biochemistry, between us earning just enough to keep us going. My thesis was on kinetics studies with the enzyme aldehyde dehydrogenase. When that was finished, I was granted a British Council Fellowship to work under the supervision of Malcolm Dixon. There, in the Department of Biochemistry at the University of Cambridge, I started a project on the mechanism of metal activation of the enzyme phosphoglucomutase. It was through that enzyme that I started to collaborate with Fred Sanger. I have described this collaboration in some detail previously (Lynen Lecture; Miami Winter Symp. Proc., In: “From gene to protein: translation into biotechnology”; Ed. W. Whelan, Academic Press, 1982). It was after completing my PhD thesis that I took a short-term appointment with the Medical Research Council in Sanger’s group, and then returned to Argentina for a period of two years. During that period I extended my studies of mechanisms of enzyme action to the enzymes phosphoglyceromutase and alkaline phosphatase. It was then that I had my first experience at directing other people’s work, including my first research student. The political persecution of liberal intellectuals and scientists manifested itself as a vendetta against the director of the institute where I was working. This forced my resignation and return to Cambridge to rejoin Fred Sanger, who by then had been appointed Head of the Division of Protein Chemistry in the newly-formed Laboratory of Molecular Biology of the Medical Research Council. Following his suggestion, I shifted my interests from enzymology to immunology. The evolution of my research in this area is described in the Lynen Lecture as mentioned above and in the Nobel Lecture.
Born 8 October 1927, in Bahía Blanca, Argentina. Married in 1953, to Celia (née Prilleltensky). No children.
| 1939-1944 | Colegio Nacional, Bahía Blanca (Bachiller) |
| 1945-1952 | Facultad de Ciencias, Universidad de Buenos Aires (Licenciado en Ciencias Químicas) |
| 1950-1956 | Part-time clinical analyst at Laboratorios Liebeschutz |
| 1952-1957 | Research Student at the Instituto de Química Biológica, Facultad de Ciencias Médicas, Universidad de Buenos Aires |
| 1957 | Doctor en Química (Universidad de Buenos Aires) |
| 1957-1963 | Staff of Instituto Nacional de Microbiología, Buenos Aires (Leave of absence 1958-1961) |
| 1958-1960 | British Council Fellowship at the Department of Biochemistry, University of Cambridge |
| 1960 | Ph.D. degree (University of Cambridge) |
| 1960-1961 | Scientific staff of Medical Research Council at the Department of Biochemistry, University of Cambridge |
| 1961-1963 | Head of División de Biología Molecular, Instituto Nacional de Microbiología, Buenos Aires |
| 1963- | Scientific Staff of Medical Research Council Laboratory of Molecular Biology, Cambridge |
| 1983 | Head, Protein and Nucleic Acid Chemistry Division, Cambridge |
Honorary member, Scandinavian Immunological Societies (1970); Member, European Molecular Biology Organization (1974); Fellow of the Royal Society (1975); Honorary member, American Association of Immunologists (1979); Fellow of Darwin College, Cambridge (1980); Honorary Fellow of Fitzwilliam College, Cambridge (1982); Foreign Associate, National Academy of Sciences, USA (1981); Honorary Fellow, Royal College of Physicians (1983); Foreign Honorary Member, American Academy of Art and Sciences (1983); Member of the Deutsche Akademie der Naturforscher Leopoldina (1983); Académico Correspondiente Extranjero of the Real Academia de Ciencias Exactas, Fisicas y Naturales, Madrid (1984).
Prizes and Awards
Prize Herrero Doucloux of the Asociación Química Argentina (1957); CIBA Medal and Prize (1978); Lewis S. Rosenstiel Award, Brandeis University (1979); Avery-Landsteiner Prize, Society for Immunology (1979); V. D. Mattia Lectureship Award, Roche Institute (1979); Adolph Rosenberg Award, University of Miami (1980); Wolf Prize in Medicine, Wolf Foundation, Israel (1980); Louisa Gross Horwitz Prize, Columbia University (1980); Robert Koch Prize and Medal, Germany (1980); Royal Society Wellcome Foundation Prize (1980); Madonnina Award, Fondazione Carlo Erba, Milano (1981); William Bate Hardy Prize, Cambridge Philosophical Society (1981); Jimenéz Díaz Memorial Award, Fundación Conchita Rabago de Jimenéz Díaz, Spain (1981); General Motors Cancer Research Foundation Sloan Prize, USA (1981); The Gairdner Foundation Annual Award, Canada (1981); Krebs Medal, Federation of European Biochemical Societies (1981); Brown-Hazen Memorial Award, Albany, New York (1982); Lynen Medal, Miami Winter Symposium (1982); Gerónimo Forteza Medal, Valencia, Spain (1982); David Pressman Memorial Award, U.S.A. (1982); Biochemical Analysis Prize 1982, German Society for Clinical Chemistry (1982); Karl Landsteiner Award, American Association of Blood Banks (1982); Royal Medal, Royal Society (1982); XI International Congress of Allergology and Clinical Immunology Award (1982); Rabbi Shai Shacknai Memorial Prize, Hebrew University, Jerusalem (1982); Philip Levine Award, American Society of Clinical Pathologists (1983); Franklin Medal, Franklin Institute, U.S.A. (1983); Mallinkrodt Award for Investigative Research, Clinical Ligand Assay Society, U.S.A. (1983); Carlos J. Finlay Prize for Meritorious Work in Microbiology, UNESCO (1983); Common Wealth Award in Science, Sigma XI Scientific Research Society, U.S.A. (1983); Dale Medal, Society for Endocrinology (1984); Albert Lasker Basic Medical Research Award, Albert and Mary Lasker Foundation (1984); John Scott Award, Board of Directors of City Trusts, Philadelphia, U.S.A. (1984).
This autobiography/biography was written at the time of the award and later published in the book series Les Prix Nobel/ Nobel Lectures/The Nobel Prizes. The information is sometimes updated with an addendum submitted by the Laureate.
César Milstein died on March 24, 2002.