Clifford G. Shull – Photo gallery
Clifford G. Shull receiving his Nobel Prize from H.M. King Carl XVI Gustaf of Sweden at the Stockholm Concert Hall on 10 December 1994.
Nobel Foundation. Photo: Lars Åström
1994 laureates on stage at the Nobel Prize award ceremony at the Stockholm Concert Hall on 10 December 1994. From left: physic laureates Bertram N. Brockhouse and Clifford G. Shull, chemistry laureate George A. Olah, medicine laureates Alfred G. Gilman and Martin Rodbell, literature laureate Kenzaburo Oe and economic sciences laureates John C. Harsanyi, John F. Nash Jr. and Reinhard Selten.
Nobel Foundation. Photo: Lars Åström
Clifford G. Shull showing his Nobel Prize diploma after the award ceremony on 10 December 1994.
Photo from the Lars Åström archive
Clifford G. Shull and Bertram N. Brockhouse with their Nobel Prizes after the award ceremony at the Stockholm Concert Hall on 10 December 1994.
Nobel Foundation. Photo: Lars Åström
Clifford G. Shull delivering his Nobel Prize lecture on 8 December 1994.
Nobel Foundation. Photo: Lars Åström
Clifford G. Shull and Bertram N. Brockhouse after delivering their Nobel Prize lectures at the Royal Swedish Academy of Sciences on 8 December 1994.
Nobel Foundation. Photo: Lars Åström
Clifford G. Shull signing books during Nobel Week in Stockholm, Sweden, December 1994.
Nobel Foundation. Photo: Lars Åström
1994 laureates assembled at the Swedish Academy in Stockholm in December 1994. Back row: Medicine laureates Martin Rodbell and Alfred G. Gilman, economic sciences laureate John F. Nash Jr., chemistry laureate George A. Olah, economic sciences laureate Reinhard Selten and physic laureate Clifford G. Shull. Front row: Physic laureate Bertram N. Brockhouse, literature laureate Kenzaburo Oe and economic sciences laureate John C. Harsanyi.
Photo from the Lars Åström archive
Bertram N. Brockhouse – Photo gallery
Bertram N. Brockhouse receiving his Nobel Prize from H.M. King Carl XVI Gustaf of Sweden at the award ceremony at Stockholm Concert Hall on 10 December 1994.
Nobel Foundation. Photo: Lars Åström
1994 laureates on stage at the Nobel Prize award ceremony at the Stockholm Concert Hall on 10 December 1994. From left: physic laureates Bertram N. Brockhouse and Clifford G. Shull, chemistry laureate George A. Olah, medicine laureates Alfred G. Gilman and Martin Rodbell, literature laureate Kenzaburo Oe and economic sciences laureates John C. Harsanyi, John F. Nash Jr. and Reinhard Selten.
Nobel Foundation. Photo: Lars Åström
Clifford G. Shull and Bertram N. Brockhouse with their Nobel Prizes after the award ceremony at the Stockholm Concert Hall on 10 December 1994.
Nobel Foundation. Photo: Lars Åström
Bertram N. Brockhouse delivering his Nobel Prize lecture on 8 December 1994.
Nobel Foundation. Photo: Lars Åström
Clifford G. Shull and Bertram N. Brockhouse after delivering their Nobel Prize lectures at the Royal Swedish Academy of Sciences on 8 December 1994.
Nobel Foundation. Photo: Lars Åström
Bertram N. Brockhouse photographed during Nobel Week in Stockholm, Sweden, December 1994.
Nobel Foundation. Photo: Lars Åström
1994 laureates assembled at the Swedish Academy in Stockholm in December 1994. Back row: Medicine laureates Martin Rodbell and Alfred G. Gilman, economic sciences laureate John F. Nash Jr., chemistry laureate George A. Olah, economic sciences laureate Reinhard Selten and physic laureate Clifford G. Shull. Front row: Physic laureate Bertram N. Brockhouse, literature laureate Kenzaburo Oe and economic sciences laureate John C. Harsanyi.
Photo from the Lars Åström archive
The Nobel Prize in Physics 1994
Press release

12 October 1994
The Royal Swedish Academy of Sciences has decided to award the 1994 Nobel Prize in Physics for pioneering contributions to the development of neutron scattering techniques for studies of condensed matter with one half to Professor Bertram N. Brockhouse, McMaster University, Hamilton, Ontario, Canada, for the development of neutron spectroscopy
and one half to Professor Clifford G. Shull, MIT, Cambridge, Massachusetts, USA for the development of the neutron diffraction technique
The structure and dynamics of matter revealed
Most people know that X-ray methods and microscopy can be used for studying objects in detail. Despite refinements these methods are not always adequate. The researchers now rewarded have developed neutron scattering techniques, powerful methods of analysing both solid and fluid (condensed) matter. The techniques were developed at the relatively simple and not-too-powerful nuclear reactors that became available to researchers shortly after the second World War. Successive developments have led to today’s large installations specially built for studies of condensed matter in, for example, France, England and the USA, and more are planned.
Both methods are based on the use of neutrons flowing out from a nuclear reactor. When the neutrons bounce against (are scattered by) atoms in the sample being investigated, their directions change, depending on the atoms’ relative positions. This shows how the atoms are arranged in relation to each other, that is, the structure of the sample. Changes in the neutrons’ velocity, however, give information on the atoms’ movements, e.g. their individual and collective oscillations, that is their dynamics. In simple terms, Clifford G. Shull has helped answer the question of where atoms “are” and Bertram N. Brockhouse the question of what atoms “do”.
Neutron scattering techniques are used in such widely differing areas as the study of the new ceramic superconductors, catalytic exhaust cleaning, elastic properties of polymers and virus structure.
Dynamic development
Brockhouse and Shull made their pioneering contributions at the first nuclear reactors in the USA and Canada as early as the 1940s and l950s. Neutron scattering techniques have since developed considerably and in the past few years neutrons have been used to an increasing extent for studying the structure (arrangement) and dynamics (movement) of solid and fluid matter. The number of researchers is now reckoned in thousands, with intensive research at the many neutron scattering installations the world over. The high flux reactor at the Institut Laue-Langevin at Grenoble, France, is an example of a large European research plant from the beginning of the 1970s (recently upgraded). Studies here include both the structure and the dynamics of the new ceramic superconductors (Nobel Prize 1987 to Bednorz and Müller), molecule movements on surfaces of relevance to catalytic exhaust cleaning, the structure of viruses and how these defend themselves against dehydration, and the connection between the ordered and the non-ordered structures of polymers and their elastic properties (Nobel Prize 1991 to de Gennes). The handbook for researchers wishing to use the installation describes no fewer than 16 instruments for studying structure and 14 for dynamics.
At the Rutherford Appleton Laboratory in England an accelerator-based neutron source (ISIS) was built for similar purposes and at NIST (the National Institute of Science and Technology) in the USA there is a 1990 variant of the Grenoble installation. It is now planned to open new and very advanced installations in Europe, the USA and Asia. Using these it is hoped to gain new basic knowledge, but also to develop technological applications (computer memory) and environmental applications (the chemistry of pollution).
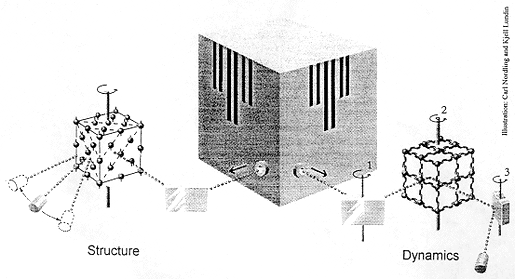
What happens
The illustration shows how neutrons from a research reactor may be used for
studying structure and dynamics.
In the left-hand part of the picture the neutron beam is first reflected in a crystal. Because of the wave nature of the neutrons – a characteristic of all moving particles -and the strict arrangement of the crystal atoms in a regular pattern, the neutrons reflected in a certain direction will have a defined wavelength (the Bragg condition). With the crystal set at a suitable angle, a certain neutron wavelength can be selected. These “monochromatised” neutrons then irradiate the sample to be investigated. Since neutrons are electrically neutral, they have great penetrability and hence search the whole sample. Most of the neutrons leave the sample with unchanged energy (elastic scattering) and a preference for certain directions (diffraction). By counting the neutrons in a rotatable detector, a diffraction pattern is obtained which shows the relative positions of the atoms in the sample. It is for the development of this variant of the neutron scattering technique that Clifford G. Shull has been awarded his Nobel Prize. He has shown how neutrons may be used to determine the atomic structure of a material.
The right-hand part of the picture shows the basic principle used by Brockhouse. Neutrons from the reactor are first monochromatised by a crystal which may be turned about an axis (1). When the neutrons penetrate the sample, which is rotatable about another axis (2), they can initiate or cancel out oscillations in the sample’s atoms. These movements, in which all atoms participate collectively, are called phonons. If the neutrons manage to create (excite) phonons, they themselves lose energy (inelastic scattering). When the neutrons have left the sample their energy is analysed in a crystal which can be turned about a third axis (3) and finally counted in a detector. Using an apparatus of this type – a triple-axis spectrometer – movements, the dynamics, of a material or a crystal may be studied. It is for the development of this technique, neutron spectroscopy for condensed matter, that Bertram N. Brockhouse has been awarded his Nobel Prize.
How it all began
At the end of the second World War researchers in the USA gained access to the large neutron fluxes that even relatively modest nuclear reactors were capable of delivering. Neutrons had then been known as building blocks in the atomic nucleus for more than a decade (Nobel Prize to Chadwick in 1935 for their discovery). Enrico Fermi showed in 1942 that neutrons from fission of the uranium nucleus could support a controlled chain reaction. He had earlier made the important discovery that slowed-down or thermal neutrons show a much greater inclination to react than fast ones do (Nobel Prize for this discovery, among others, to Fermi in 1938). It is the special properties of these slow neutrons that make them suitable for detecting the positions and movements of atoms. Even before the entry of the nuclear reactors into the research arena, results of using simple neutron sources had indicated that neutron beams could be used for studying solid bodies and liquids. However, there were many difficulties to be overcome before these possibilities could be realised.
At the nuclear reactor at Oak Ridge in the USA the late E.O.Wollan formed a working group to examine the possibilities of developing neutron beams and apparatuses for determining structure. Clifford Shull was early linked to this group and was soon to play a major part. Similar efforts were being made elsewhere but it was the Wollan-Shull group and later Shull in collaboration with other researchers, that proceeded most purposively and achieved results with surprising rapidity. Shull’s studies of simple crystals laid a basis for the interpretation of the very complicated structures being analysed by modern neutron crystallographers.
Where are the hydrogen atoms?
Hydrogen is one of the commonest elements in biological matter. It also occurs in many forms of technically important inorganic matter. The localisation of hydrogen in the structures of these would have been practically impossible with the earlier X-ray diffraction method (for which von Laue and the Braggs, father and son, became Nobel laureates in 1914-1915) since the hydrogen atom gives a scarcely noticeable scattering of X-ray radiation. (X-ray beams are scattered against electrons in the diffracting atoms, and the hydrogen atom has only one electron). As opposed to this, the nucleus of the hydrogen atom, the proton, constitutes a very efficient neutronscattering centre and its position can therefore be determined with neutron diffraction. Through his first successful experiments Shull opened what was to become a very large field for finding out how hydrogen is bound in, for example, ice, metallic hydrides and organic compounds.
Magnetic structures
Neutrons are small magnets, as are the atoms of a magnetic material. When a neutron beam strikes such material, the neutrons can therefore change direction through magnetic interaction with the atoms of the material. This gives rise to a new type of neutron diffraction (the type described earlier is based on neutron interaction with atomic nuclei) which can be used to study the relative orientations of the small atomic magnets. Here, too, the X-ray method has been powerless and in this field of application neutron diffraction has since assumed an entirely dominant position. It is hard to imagine modern research into magnetism without this aid.
A new breakthrough
While Shull was developing the neutron scattering technique based on the diffraction of elastically scattered neutrons, Brockhouse at the Chalk River research reactor, in Canada, was concentrating chiefly on inelastic scattering. He designed the triple-axis spectrometer already mentioned and developed methodology for studying the energy spectrum of the neutrons once they had been scattered. This required profound knowledge of the properties of neutrons and great ingenuity. It was only with Brockhouse’s contributions that inelastic neutron scattering became a useful tool in the physics of condensed matter. Neutrons again proved to have unique scattering properties, in this case because their energy is of the same order of magnitude as that of the phonons in solid and fluid matter. During a hectic period between 1955 and 1960 Brockhouse’s pioneering work was without parallel within neutron spectroscopy. This enabled the technique to develop into an in many ways unique source of information which has revolutionised our ability to chart atomic dynamics, e.g. atomic vibrations in crystals, diffusion movements in liquids and fluctuations in magnetic material. Such information is contributing actively to the elucidation of the forces that bind atoms to one another in solid bodies and that determine, for instance, the transition from the solid state to the fluid state.
Phonons and magnons
The number of atoms in a macroscopic quantity of matter is very large, giving rise to a rich flora of movement types in solid and fluid bodies. The connection between energy and wavelength in, for example, crystal oscillations, termed the phonon dispersion relation, is a complicated function. The shape of the dispersion curve is, however, characteristic for a crystal, and mapping this affords valuable information on the properties of materials. As early as in 1955 Brockhouse and A.T. Stewart reported results concerning phonons in, among other things, aluminium crystals, and they specified for the first time an experimentally measured dispersion curve for these.
In crystals of magnetic material, e.g. magnetite, a type of collective wave motion can occur among the atomic elementary magnets. This wave motion can be excited by neutrons, and Brockhouse was the first to study and measure the dispersion curve for the elementary excitations of this wave motion, termed magnons.
Non-ordered movement
For non-ordered movement in fluids and melts, as in magnets, the late L. Van Hove formulated, in the early 1950s, a theory of how the memory of a certain arrangement of atoms, gradually disappears over time. Neutrons make it possible to follow the change of atomic structures over time. Brockhouse was the first to show experimentally how these ‘correlation’ or ‘memory’ functions could be determined using neutron scattering in experiments with, among other substances, H2O (water) and D2O (heavy water). In the same way, his experiments with liquid lead provided a model for others to follow.
Such experiments provided the starting point for a lively development of theories for liquids and non-ordered systems in general. Phenomena such as lattice dynamics and diffusion underwent a renaissance through these and subsequent research contributions.
Through the studies of atomic structure and dynamics made possible by Bertram N. Brockhouse and Clifford G. Shull with their development of neutron scattering techniques, valuable information is being obtained for use in e.g. the development of new materials. An important example is the ceramic superconductors now being studied intensively, although these have not yet been developed for commercial use.
Clifford G. Shull – Biographical

I was born on September 23, 1915 to my parents, David H. and Daisy B. Shull, in the section of Pittsburgh, Pennsylvania, known as Glenwood, which obviously relates to their selection of my middle name. I was preceded by an older sister, Evalyn May, and an older brother, Perry Leo, so that I grew up as the baby in the family. Both my father and mother had origins in rural, central Pennsylvania, in farming sections of Perry County. After moving with his then family to the big city, Pittsburgh, my father started a small business that evolved into a hardware store and an associated home repair service.
My early years of growth were entirely normal and happy ones and I had the usual collection of friends and buddies, who were often seen on the ball field or on roller skates. Grade schooling was nearby, a few blocks from our home, and this led later on to junior high school in the adjoining Hazelwood section but still within walking distance of our home. Following this, I had decided to go to Schenley High School for the remaining three years of school work and this required a more troublesome commute of 45 minutes by public street car. My first interest in physics as a career speciality came during my senior year at Schenley when I took the physics course taught by Paul Dysart. Somewhat older than the usual high school teacher and with a PhD degree in his background, he was a very impressive teacher who delighted in demonstrations from his laboratory and in explaining the principles behind them. Thereafter my original interest in aeronautical engineering was in heavy competition with physical science.
It seemed natural, in view of limited family financial straights, that I should continue into college study by living at home and commuting to the Carnegie Institute of Technology (now Carnegie Mellon University). Carnegie Tech was also located in the Schenley Park district of Pittsburgh so that essentially the same commute was called for and it offered good, reputable curricula in the engineering and physical sciences. I was pleased when offered admission to the fall term of 1933 and particularly so when given a half-tuition scholarship in view of my good high school record. Once there, my interest in physics as a major subject sharpened quckly, helped along no doubt by the brilliant lectures in my freshman physics course given by Harry Hower, the chairman of the Physics Department. Hower was more aptly labeled an optical and illuminating engineer than a physicist, because of his extensive consulting activities in coastal lighthouse lens design and other architectural problems, but his lectures were delightful, inspiring and not often-to-be-missed by his students.
Shortly after my admission at Carnegie Tech, a family crisis developed when my father died unexpectedly in January, 1934. By this time, my sister had married and, with her husband, were living at home along with my brother (who had just finished college as an art major), my mother and myseelf. My brother decided to forego his art teaching and operate my father’s business and this continued until I had finished Carnegie Tech in 1937. The four years spent there were entirely pleasurable ones, in spite of the time-consuming commute, and I enjoyed the association with my fellow students and professors in the department. I was able to work in the summer periods at jobs both on and off campus and this helped to meet my rather minimal expenses during the year. Among the professors, I valued very much the friendly encouragement and counsel offered by Emerson Pugh during my junior and senior years, leading to my continuance into graduate school at New York University in the fall of 1937.
New York University was then a very large university, perhaps the largest in the nation, with several distributed, more or less autonomous, campuses. I was located with the Physics Department at the University Heights campus in the upper Bronx section of New York City and my teaching assistantship provided living subsistence, teaching meaning laboratory course help and problem assignment grading. We graduate students were encouraged at an early stage to join and help in one of the half dozen or so ongoing research projects within the department. I became associated with a nuclear physics group headed by Frank Myers and Robert Huntoon, who were in the process of building a 200 keV Cockcroft-Walton generator for accelerating deuterons. Much valuable experience was obtained with this exposure by Craig Crenshaw, another graduate student, and myself and we were able to help in the initial experiment with this accelerator, a study of the D-D nuclear reaction.
During the third year of my graduate study, the Department decided that it could support the construction of a new 400 keV Van de Graaff generator to be used for accelerating electrons. Frank Myers took on this responsibility with me as his assistant and the thought that it could be used to repeat the electron-double-scattering (EDS) experiment as a possible thesis topic for me. This EDS type of experiment loomed important at the time because it was considered a direct test that electrons have a spin or polarization. Several earlier experiments had given either negative or inconclusive results and it seemed worthwhile that the experiment be performed again under new conditions. The construction and testing of the new facility went smoothly and I turned to getting ready for my thesis EDS experiment. By this time, Frank Myers had decided to take his overdue sabbatical leave with Robert Van de Graaff at MIT. I was fortunate in getting Richard Cox, a senior professor in the department, to supervise and offer expert and friendly advice on my efforts. Finally after four months of data collection and analysis, the experiment was successful and I was able to prepare a thesis and take my PhD degree in June 1941.
Among the other research programs being pursued by the NYU department was the study of neutron interactions with materials as started by Alan Mitchell and carried on by Martin Whitaker. Using a Ra-Be neutron source surrounded by a paraffin howitzer, a modest beam of thermalized neutrons was available for experimentation and, during my period at the Heights, this was directed towards a search for the expected paramagnetic scattering from certain materials. Theoretical prediction of this had been given by O. Halpern and M. Johnson and their students in the Department. I was familiar with this problem through my contemporary graduate student William Bright who worked with Whitaker on the experiment and indeed found myself working on the same problem a decade later.
I have neglected to mention an important event that occurred in my first year in New York City. Through my good friend Craig Crenshaw, I was introduced to a young lady, Martha-Nuel Summer, who had recently come from South Carolina to the graduate school at Columbia University to study early American History. Our friendship flourished during the years of our professional studies and we married shortly after I took my degree and had a job in waiting. She has remained my loving companion to the present and along the way we have been favored by three fine sons, John, Robert and William, who have beautiful families of their own.
I had arranged for a position at Beacon, NY with the research laboratory of The Texas Company, and Martha and I set up housekeeping there in July 1941. This laboratory addressed problems associated with the production and use of petroleum fuels and lubricants and included a small group of physicists. I was asked to study the microstructure of catalysts using gas adsorption and x-ray diffraction and scattering as tools for characterizing the physical structure of these materials. These catalysts were used in the production of high-performance aviation fuel and this area of investigation became increasingly important after the US entry in the World War in December 1941. Of singular significance to the scientific community in the first year of our wartime activity was the growth of the Manhattan Project dealing with the development of an atomic weapon. Many scientists had been drawn into this, including a number of my old colleagues and professors from graduate school. I was encouraged to join them and would have done so except that The Texas Company would not agree to my wartime job change. The matter was finally settled in their favor by an adjudication hearing at an area manpower board and I stayed in Beacon through the war years.
My work at Beacon was interesting and challenging and gave me the opportunity of learning things about diffraction processes, crystallography and the new field of solid state physics. Through visits and early meetings of the American Society for X-ray and Electron Diffraction, I was able to know established personages such as Warren, Buerger, Fankuchen, Zachariasen, Ewald, Harker, Gingrich and Donnay. Once the war was over, my interest in participating in the exciting new developments in nuclear physics within the Manhattan Project returned, and I paid a visit to the Clinton Laboratory (now Oak Ridge National Laboratory) in Tennessee. The activity there fascinated me very much and I convinced Martha that we should move there, which we did in June 1946 along with our one and a half year old son.
It was arranged that I would work with Ernest Wollan, who had been at the Laboratory since its formation during the war period and who had just assembled a rudimentary two-axis spectrometer for obtaining neutron diffraction patterns of crystals and materials. Wollan had shown me his first powder diffraction pattern on my earlier visit and I was delighted to be able to join him in exploring how neutron patterns could be used to supplement those obtained with x-rays or electrons. Our collaboration on common problems was to continue for nearly a decade until I left Oak Ridge in 1955 for academic life at Massachusetts Institute of Technology. I regret very much that Wollan’s death in 1984 precluded his sharing in the Nobel honor that has been given to Brockhouse and me since his contributions were certainly deserving of recognition.
I was attracted to MIT by the prospects of teaching and of training graduate research students at the soon-to-be-completed MITR-I research reactor on campus. This reactor was among the early group of condensed volume reactors using isotopically enriched fuel which were being explored in that period. Together with occasional post-doctoral students and a regular flow of graduate thesis students, our group carried on investigations using neutron radiation from this reactor in many fields until my retirement from MIT in 1986. These studies included: internal magnetization in crystals, development of polarized beam technology, dynamical scattering in perfect crystals, interferometry, and fundamental properties of the neutron. The opportunity of being at MIT with its fine faculty and excellent students has certainly been most stimulating and satisfying.
This autobiography/biography was written at the time of the award and later published in the book series Les Prix Nobel/ Nobel Lectures/The Nobel Prizes. The information is sometimes updated with an addendum submitted by the Laureate.
Clifford G. Shull died on March 31, 2001.
Bertram N. Brockhouse – Curriculum Vitae
| BERTRAM NEVILLE BROCKHOUSE: Born July 15, 1918 at Lethbridge, Alberta, Canada |
| Son of Israel Bertram and Mable Emily (Neville) Brockhouse |
| Educated: King George High School, Vancouver, B.C. |
| Served in the Royal Canadian Navy 1939-1945 |
| B.A. 1947 (Mathematics and physics) University of British Columbia |
| M.A. 1948 and Ph.D. 1950 (Physics: Solid State) University of Toronto |
| Married to Doris Isobel Mary Miller 1948 |
| Research Officer, Chalk River Laboratory, 1950-1959 |
| Seconded for 10 months to Brookhaven National Laboratory 1953-54 |
| Head, Neutron Physics Branch CRNL, 1960-1962 |
| Member of Council, Canadian Association of Physicists, 1960-61 |
| Fellow, Royal Society of Canada, 1962 Oliver S. Buckley Prize of the American Physical Society, 1962 |
| Professor of Physics, McMaster University, 1962-1984 |
| Duddell Medal and Prize of the Institute of Physics, 1963 |
| Fellow, Royal Society of London, 1965 Chairman, Department of Physics, McMaster University, 1967-70 |
| Medal for Achievement in Physics, Canadian Association of Physics, 1967 |
| Centennial Medal of Canada, 1967 |
| Doctor of Science, Honoris Causa, University of Waterloo, 1969 |
| Member of Council, American Physical Society, 1969-1973 |
| Sabbatical year (1970-71 – Guggenheim Fellowship – BNL, ORNL in USA, AERE Harwell, England) |
| Tory Medal of the Royal Society of Canada, 1973 |
| Memberships in societies for History and Philosophy of Science Officer, The Order of Canada, 1982 |
| Retired in 1984. Professor Emeritus of Physics, McMaster University |
| Doctor of Science, Honoris Causa, McMaster University, 1984 |
| Foreign Member, Royal Swedish Academy of Sciences, 1984 |
| Honorary Foreign Member, American Academy of Arts and Sciences, 1990 |
| Nobel Prize in Physics, 1994 (with C.G. Shull) |
| some 90 publications, mostly in neutron physics |
| six children, eight grandchildren. |
This CV was written at the time of the award and later published in the book series Les Prix Nobel/Nobel Lectures. The information is sometimes updated with an addendum submitted by the Laureate. To cite this document, always state the source as shown above.
Bertram N. Brockhouse died on October 13, 2003.
Bertram N. Brockhouse – Nobel Lecture
Nobel Lecture, December 8, 1994
Slow Neutron Spectroscopy and the Grand Atlas of the Physical World
Read the Nobel Lecture
Pdf 644 kB
Clifford G. Shull – Nobel Lecture
Nobel Lecture, December 8, 1994
Early Development of Neutron Scattering
Read the Nobel Lecture
Pdf 323 kB
Clifford G. Shull – Other resources
Links to other sites
‘Clifford Shull, Neutron Diffraction, and Neutron Scattering’ from DOE R&D Accomplishments
Clifford G. Shull – Banquet speech
Clifford G. Shull’s speech at the Nobel Banquet, December 10, 1994
Your Majesties, Your Royal Highnesses, Ladies and Gentlemen,
I need not say that Bertram Brockhouse and I are immensely grateful to the Royal Swedish Academy of Sciences for affording us the great honor of being named Laureates today. Being selected as such is a dream for any scientist who hopes that his work will prove useful to others.
If one looks back through the history of advances in physics, you find many examples of notable findings from scattering experiments. In this, you introduce some entity to a target assembly and upon studying its interaction with the target you learn much about the latter. Over the last century, physicists have used light quanta electrons, alpha particles, X-rays, gamma-rays, protons, neutrons and exotic sub-nuclear particles for this purpose. Much important information about the target atoms or nuclei or their assemblage has been obtained in this way. In witness of this importance one can point to the unusual concentration of scattering enthusiasts among earlier Nobel Laureate physicists. One could say that physicists just love to perform or interpret scattering experiments.
And that is where Bert Brockhouse and I came into the picture. We were separately introduced to intense beams of low energy neutrons, recently available from World War-period devices, called nuclear reactors, in the years following the war at places separated by a thousand miles in separate research surroundings. Our challenge then was to see what neutrons could be used for, considering their unique characteristics.
The wonderful reward we have received today seems to indicate that some of nature’s secrets are indeed vulnerable to this tool of the physicist. We are indeed appreciative of the honor. Thank you very much.