Herbert C. Brown – Photo gallery
Professor Herbert C. Brown in his lab. (Undated and unknown location)
Credit: Purdue University
Professor Herbert C. Brown and his son, Charles Brown, work on an early version of the Brown Autogasimeter.
Credit: Purdue University
Portrait of Herbert C. Brown. Photo taken in 2005.
Credit: Purdue University
Professor Herbert C. Brown in his lab at Purdue University.
Credit: Purdue University
In the mid-1990s, postdoctoral researcher P.V. Ramachandran and Professor Herbert C. Brown monitor a reaction using 11B NMR spectroscopy inside the NMR room of Wetherill Lab at Purdue University.
Credit: Purdue University
Georg Wittig – Nobel Lecture
Nobel Lecture, December 8, 1979
From Diyls to Ylides to My Idyll
Read the Nobel Lecture
Pdf 106 kB
Herbert C. Brown – Nobel Lecture
Nobel Lecture, December 8, 1979
From Little Acorns to Tall Oaks – from Boranes through Organoboranes
Read the Nobel Lecture
Pdf 335 kB
Herbert C. Brown – Other resources
Links to other sites
Herbert C. Brown’s page at Purdue University
Biographical memoires from National Academy of Sciences
Herbert C. Brown – Biographical

My parents, Charles Brovarnik and Pearl Gorinstein, were born in Zhitomir in the Ukraine and came to London in 1908 as part of the vast Jewish immigration in the early part of this century. They were married in London. In 1909 my sister, Ann, was born. I arrived on May 22, 1912. In June 1914 my father decided to join his mother and father and other members of his family in Chicago, much to the dismay of my mother, whose own family largely remained in England. My grandfather’s name had been anglicized to Brown, and that became our name. In the United States, my two sisters, Sophie and Riva, were born in 1916 and 1918.
My father had been trained as a cabinet maker, doing delicate inlaid work. However, he found little market for his skills in the U.S. and turned to carpentry.
The Depression of 1920 persuaded him to go into business and he opened a small hardware store in Chicago at 18th and State Street, largely a black neighborhood. We lived in an apartment above the store and I attended the Haven School at Wabash and 16th Street with predominantly black classmates.
I did well in school and was advanced several times, graduating at 12. Indeed, I was offered, but refused, further advancement since I did not want to be in the same class with my sister, Ann.
On graduation, I went to Englewood High School on the South Side of Chicago. Unfortunately, my father became ill of some sort of infection and died in 1926. I left school to work in our store. I am afraid that I was not really interested in the business and spent most of my time reading. My mother finally decided that she would attend the store and I should go back to school. Accordingly, I reentered Englewood in February 1929 and graduated in 1930.
At Englewood I ran the humor column of the school paper and won a national prize. I never recovered.
We sold the store at that time. I had no hope of going on to college. However, this was the beginning of the Depression and I could find no permanent job. Studying appealed to me much more than the odd jobs I could find. I decided to go to college. I entered college intending to major in electrical engineering. I had heard that one could make a good living in that area. However, I took chemistry and became fascinated with that subject, and remained with chemistry thereafter. I had just completed one semester at Crane Junior College when it was announced in 1933 that the school was to be closed for lack of funds. I then went to night school at the Lewis Institute, taking one or two courses, financing myself by working as a part-time shoe clerk.
I then heard that one of the instructors at Crane, Dr. Nicholas Cheronis, had opened his laboratory to several students, so that they could continue their studies on their own. I went there and grew to know and love a fellow student, Sarah Baylen. Sarah had been the brightest student in chemistry at Crane prior to my arrival. She has described (“Remembering HCB”) how she initially “hated my guts.” But since she could not beat me, she later decided to join me, to my everlasting delight.
In 1934 Wright Junior College opened its doors. We went there and nine of us graduated in 1935 as the first graduating class. In my yearbook Sarah predicted that I would be a Nobel Laureate!
I had been advised to take the competitive examination for a scholarship at the University of Chicago. I did so. To my astonishment, little of the examination was devoted to the chemistry, physics, and mathematics that had constituted the major portion of my studies. Instead, the examination emphasized general subjects: history, art, music, literature, etc. – subjects I had never studied formally. I did the best I could and was pleasantly surprised when I received a half scholarship.
I entered the University of Chicago in the Fall of 1935, accompanied by my girlfriend, Sarah. This was the time when the President of the University, Robert Maynard Hutchins, was arguing for the principle that students should be permitted to proceed as rapidly as possible. Indeed, at that time it cost no more to take ten courses than it did the usual three. I did so, and completed my junior and senior year in three quarters, receiving the B.S. in 1936.
.
I did not apply for graduate work. I wanted to find a job and marry my girlfriend. However, a famous organic chemist, Julius Stieglitz – then Emeritus, but still teaching – called me into his office and urged me to reconsider my decision. He predicted a favorable future as a research chemist. I discussed the matter with Sarah and she agreed that marriage could wait. Accordingly, I began graduate work.
On my graduation, Sarah presented me with a gift – a copy of Alfred Stock’s book, The Hydrides of Boron and Silicon. This book interested me in the hydrides of boron and I undertook to study with Professor H.I. Schlesinger, then active in that area of research.
Sarah and I were married “secretly” on February 6, 1937. We were such innocents that we did not realize that marriages are published in the daily newspapers. Consequently, our marriage was a secret for the weekend!
Once the news got out, I had to begin supporting her. But my income as a graduate assistant was only $400 per year, out of which had to come $300 for tuition. But Sarah obtained a position at Billings Hospital in Medical Chemistry and kept us solvent.
I received my Ph.D. in 1938. Unfortunately (perhaps fortunately), I could not find an industrial position. Professor M.S. Kharasch then offered me a position as a postdoctorate at a stipend of $1600 and my academic career was initiated. The following year Professor Schlesinger invited me to become his research assistant with the rank of Instructor, replacing Anton B. Burg, who was moving on to the University of Southern California. Consequently, I am an unusual example of a chemist who ended up in academic work because he could not find an industrial position.
At that time one did not achieve tenure until after ten years. I had seen a number of individuals who had remained at Chicago as Instructors for nine years without tenure and then had to find another position under severe pressure. I decided to avoid this situation. Accordingly, after four years I asked Professor Schlesinger for a decision as to my future in the Department. When he came back with the word that there was no future, I undertook to find another position.
Fortunately, Morris Kharasch had a good friend, Neil Gordon, who had just gone as Department Head to Wayne University in Detroit. (Neil Gordon, the originator of the Gordon Research Conferences, had given Morris Kharasch his first position at the University of Maryland back in 1920.) Neil Gordon was persuaded to give me a position at Wayne as Assistant Professor, preserving my academic career. I became Associate Professor in 1946, and was invited to Purdue in 1947 by the Head of the Chemistry Department, Henry B. Hass, as Professor of Inorganic Chemistry. In 1959 I became Wetherill Distinguished Professor and in 1960 Wetherill Research Professor. I became Emeritus in 1978, but continue to work with a large group of postdoctorates.
Originally my research covered physical, organic and inorganic chemistry and I took students in all three areas. However, as the Department became more organized into divisions, it became necessary to make a choice, and I elected to work primarily with coworkers in organic chemistry.
In addition to my research program in the borane-organoborane area, described in my Nobel Lecture, my research program has involved the study of steric effects, the development of quantitative methods to determine steric strains, the examination of the chemical effects of steric strains, the non-classical ion problem, the basic properties of aromatic hydrocarbons, a quantitative theory of aromatic substitution, and the development of a set of electrophilic substitution constants, s+, which correlate aromatic substitution data and a wide variety of electrophilic reactions.
Recognitions
Professor Brown was the Harrison Howe Lecturer in 1953, the Centenary Lecturer of The Chemical Society (London) in 1955, and the Baker Lecturer in 1968. He was elected to the National Academy of Sciences in 1957, the America n Academy of Arts and Sciences in 1966, received an honorary Doctorate of Science degree from the University of Chicago in 1968 and was elected Honorary Fellow of The Chemical Society and Foreign Member of the Indian National Academy of Sciences in 1978. Finally, he is the recipient of the Nichols Medal for 1959, the ACS Award for Creative Research in Synthetic Organic Chemistry for 1960, the Linus Pauling Medal for 1968, the National Medal of Science for 1969, the Roger Adams Medal for 1971, the Charles Frederick Chandler Medal for 1973, the Madison Marshall Award for 1975, the CCNY Scientific Achievement Award Medal for 1976, the Allied Award for 1978, the Ingold Memorial Lecturer and Medal for 1978, the Elliott Cresson Medal for 1978, and the Nobel Prize for 1979.
This autobiography/biography was written at the time of the award and later published in the book series Les Prix Nobel/ Nobel Lectures/The Nobel Prizes. The information is sometimes updated with an addendum submitted by the Laureate.
Herbert C. Brown died on December 19, 2004.
Georg Wittig – Facts
Press release

15 October 1979
The Royal Swedish Academy of Sciences has decided to award the 1979 Nobel Prize in Chemistry jointly to
Professor Herbert C Brown, Purdue University, West Lafayette, Indiana, USA and
Professor Georg Wittig, University of Heidelberg, Federal Republic of Germany
for their development of the use of boron- and phosphorus-containing compounds, respectively, into important reagents in organic synthesis.
Nobel Prize in chemistry shared for work in organic synthesis
In contrast to most other natural sciences chemistry is not exclusively restricted to the study of Nature per se. Chemists can combine atoms into substances which do not exist in Nature. This is particularly true for organic chemistry, the chemistry of carbon compounds, where the possibilities for preparing new compounds are virtually unlimited. These possibilities have stimulated the development of chemistry and the practical consequences have been enormous.
The chemist soon learned to make dye stuffs which were both cheaper and better than naturally occurring dyes. Chemical explosives such as nitroglycerine were developed and their connection with the Nobel Prize is well known. Synthetic drugs have saved innumerable lives and spared mankind a great deal of suffering. The synthesis of vitamins and essential amino acids, used as food additives, have improved our diet, especially in developing countries. Plastics, which are a part of our daily lives, are products of organic synthesis on a large scale. Chemicals for controlling microorganisms, insects and weeds have saved millions of lives and reduced starvation. Many such substances, however, have long-lasting effects which cannot be accepted and chemists and biologists are trying to develop better preparations.
Access to efficient synthetic reactions has been a prerequisite for these developments and new synthetic methods have been developed during tile past 150 years. Some of these nethods have had such an impact that their originators have been awarded the Nobel Prize.
Boron compounds
Herbert C Brown, who is professor at Purdue University, has developed new reagents containing boron. He jokes about his initials H, C, and B, which are also the chemical symbols for hydrogen carbon, and boron, the elements contained in the compounds which he studies. One of his reagents is sodium boro-hydride, which has become the reagent of choice for reduction of carbonyl compounds (Fig 1). He has also modified the boro-hydrides into reagents for highly selective chemical transformations.
In addition Brown has introduced an entirely new class of compounds, the organoboranes, obtained by reacting diborane with olefins (Fig 2).
Thanks to the work of Brown and his coworkers, the organoboranes have become the most versatile reagents ever created in organic chemistry. They can be used for reductions, rearrangements and additions and have opened up a range of new possibilities for linking carbon atoms to each other.
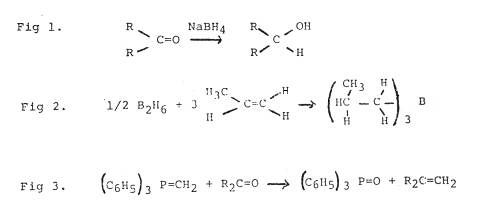
The Wittig reaction
Georg Wittig has been professor in Freiburg, Tübingen and Heidelberg, and is now professor emeritus. He has developed new synthetic methods of considerable importance and has studied reaction mechanisms. His most important achievement is the discovery of the rearrangement reaction which bears his name. In the Wittig reaction (Fig 3) an organic phosphorus compound with a formal double bond between phosphorus and carbon is reacted with a carbonyl compound. The oxygen of the carbonyl compound is exchanged for carbon, the product being an olefin. This method of making olefins has opened up new possibilities, not the least of which are for the synthesis of biologically active substances containing carbon-to-carbon double bonds. For example, vitamin A is synthesized industrially using the Wittig reaction.
Brown’s and Wittig’s results have opened up new vistas in organic synthesis and highly stimulated the further development of their science. Their methods were rapidly introduced not only into chemical laboratories, but also into elementary text books and laboratory courses.
Award ceremony speech
Presentation Speech by Professor Bengt Lindberg of the Royal Academy of Sciences
Translation from the Swedish text
Your Majesties, Your Royal Highnesses, Ladies and Gentlemen,
Chemistry is a natural science which is not entirely devoted to the study of natural objects. The art of chemistry also includes the ability on the part of the chemists to prepare or synthesize various chemical compounds. This is especially true for carbon compounds, the chemistry of which is called organic chemistry. Carbon atoms may be linked to other carbon atoms and there appears to be no limit to how many times this may be repeated in a molecule. The possibilities for variation therefore are immense. Chemists have synthesized more than two million organic compounds. These possibilities to synthesizing compounds have greatly enriched chemistry and have had enormous practical consequences.
Through the use of synthetic pharmaceuticals, vitamins and pesticides against microorganisms, insects and weeds, millions of lives have been saved, much suffering has been alleviated and world famine has been reduced. Further significant progress may be expected in a range of practical, important areas, especially concerning the development of specific pesticides less disturbing to the environment than the present ones.
One of the most important tasks for the present-day organic chemists, in basic as well as in applied research, is the synthesis of biologically active compounds. In order to achieve this, we need methods of joining carbon atoms to one another and to modify organic compounds in a variety of ways. A great many chemists devote their time to developing such methods. A few times in the history of chemistry have new synthetic methods been deemed so important that the originators have been awarded the Nobel Prize. This has once more happened, this year Brown and Wittig have been awarded the prize for chemistry for their development of boron and phosphorus compounds, respectively, into important reagents in organic synthesis.
Herbert C. Brown has systematically studied various boron compounds and their chemical reactions. He has shown how various specific reductions can be carried out using borohydrides. One of the simplest of these, sodium borohydride, has become one of the most used chemical reagents. The organoboranes, which he discovered, have become the most versatile reagents in organic synthesis. The exploitation of their chemistry has led to new methods for rearrangements, for addition to double bonds and for joining carbon atoms to one another.
Georg Wittig has provided many significant contributions in organic chemistry. The most important of these is the discovery of the synthetic method which bears his name, the Wittig reaction. In this, phosphorus ylides, a type of compound which he discovered, are allowed to react with carbonyl compounds. An exchange of groups takes place and the result is a compound in which two carbon atoms have been joined by a double bond. Since many natural products with biological activity contain such bonds, this elegant method has found wide-spread use, for example in the industrial synthesis of vitamin A.
Professor Brown,
Your discovery and exploration of borohydrides and organo boranes have given the chemists new and powerful tools for organic syntheses. May I convey to you the warmest congratulations of the Royal Swedish Academy of Sciences.
Professor Wittig,
Die Reaktion die alle, aber nicht Sie selber, die Wittig Reaktion nennen, ist eine der wichtigsten Reaktionen der organischen Chemie geworden. Sie ist besonders geeignet für die Synthese verschiedener biologisch aktiver Moleküle. Ich überbringe Ihnen die herzlichsten Glückwünsche der Koniglichen Schwedischen Akademie der Wissenschaften.
Professor Brown, Professor Wittig, may I ask you to receive the Nobel Prize for Chemistry from the hands of His Majesty the King.
Speed read: Chemical construction tools
To artificially create carbon-based compounds relies on outside help to facilitate the many ways in which carbon atoms can join onto each other and other atoms. The tools of the trade are a host of chemicals, or reagents, which take part in reactions that piece together the correct molecules in the correct manner in a step-by-step manner, either by binding them together in the right way or modifying them to provide a useful intermediate step for creating the final product. The two recipients of the 1979 Nobel Prize in Chemistry added valuable reagents to the chemical assembly toolbox by taking unfashionable elements in chemistry and showing how they interact with carbon atoms in unexpected, and as it turns out very useful, ways.
A book he received as a graduation present provided Herbert Brown with the inspiration for studying boron, an element investigated by few chemists. His fascination with how this element attaches itself to other elements like hydrogen and carbon led him to systematically study a range of boron-containing compounds, rigorously piecing together the step-by-step way in which they carry out chemical reactions. By pursuing particularly unusual looking reactions or those that did not meet their expected outcomes, Brown discovered that the chemical sodium borohydride, under specific reaction conditions, could affect carbon atoms in previously unseen ways, rearranging them or providing a useful intermediate for creating bonds between carbon and other elements, such as oxygen or nitrogen. As a result, sodium borohydride became one of the most versatile reagents chemists have at their disposal, providing a range of new possibilities for linking carbon atoms to each other when synthesizing various chemicals.
Georg Wittig also studied the course of reactions, in his case involving chemicals that contain the dangerous and explosive element phosphorus. His investigations of a class of compounds he discovered called ylides showed that a peculiar exchange takes place when they react with compounds containing carbon and oxygen, resulting in a product that contains the type of double bond between two carbon atoms that exists naturally in many important compounds. Chemists had other ways for creating double bonds, but this reaction, later named after Wittig, soon became a standard tool in creating artificial compounds, as it allowed them to introduce carbon-carbon double bonds at precise locations conveniently and effectively.