Henry Taube – Prize presentation
Henry Taube – Banquet speech
Henry Taube’s speech at the Nobel Banquet, December 10, 1983
Your Majesties, Your Royal Highnesses, Ladies and Gentlemen:
I have the honor to represent the science professed by Alfred Nobel, an honor enhanced by the presence here of so many of the Nobel family.
My friend professor Lindqvist earlier today referred to chemistry as a rapidly aging science, a remark in apparent conflict with what I had intended as my opening statement, namely that chemistry as a science is still in its infancy. I hold to my view because there is still so much beyond our understanding even in the simplest systems the chemist has cared to deal with. It should be noted that there is no real conflict in the two statements: because a child of one doubles its age after the passage of a single year, it can be said to be aging rapidly.
Undeveloped though the science is, it already has great power to bring benefits. Those accruing to physical welfare are readily recognized, as in providing cures, improving the materials needed for everyday living, moving to ameliorate the harm which mankind by its sheer numbers does to the environment, to say nothing of that which even today attends industrial development. And as we continue to improve our understanding of the basic science on which applications increasingly depend, material benefits of this and other kinds are secured for the future.
But the benefits of science are not to be reckoned only in terms of the physical. Science as an intellectual exercise enriches our culture, and is in itself ennobling. Who can fail to be uplifted by the kind of vision that the laureates in physics have provided into the outer reaches of space? Though to the layman, the world revealed by the chemist may seem more commonplace, it is not so to him. Each new insight into how the atoms in their interactions express themselves in structure and transformations, not only of inanimate matter, but particularly also of living matter, provides a thrill.
This joy of discovery is real, and it is one of our rewards. So too is the approval of our work by our peers. At a modest level we experience it in the glow we feel when a paper we write wins the unanimous approval of the referees. But approval at the exalted level signified by this occasion can be unsettling.
It is reassuring then to remember who the laureates of the past are. They are all of the utmost distinction; the selection process has worked. Reassuring to me too were the messages of congratulations from co-workers and from other experts in the field of my research. I want to thank them all, co-workers and fellow workers, for the contributions they have made, which have made my own possible, and for sentiments so generously expressed on the occasion of this award.
Reassuring, and gratifying as well, is the thought that the award recognizes a subject – the study of the reactivity of metal ion complexes – the birth of which I have witnessed and which I have helped to nurture. It too is still in its infancy; it too is flourishing. As basic science it is bound to bring benefits. That direct benefits from it may be realized during my lifetime is a hope I cherish.
No member of my family nor I can possibly forget this beautiful occasion. Nor can we forget the warmth of our reception in this community. For the opportunity to participate here, and for your hospitality, I thank you from the bottom of my heart.
Henry Taube – Other resources
Links to other sites
‘Henry Taube and Coordination Chemistry’ from DOE R&D Accomplishments
Henry Taube – Biographical

| Born: Neudorf, Saskatchewan, Canada, 1915; naturalized U.S. citizen, 1942. |
| Education |
| B.S.: 1935, University of Saskatchewan |
| M.S.: 1937, University of Saskatchewan (Research Supervisor, Prof. J.W.T. Spinks) |
| Ph.D.: 1940, University of California, Berkeley (Research Supervisor, Prof. W.C. Bray) |
| Professional Experience |
| Instructor, University of California, Berkeley, 1940-41 |
| Instructor and Assistant Professor, Cornell University, 1941-46 |
| Assistant Professor, Associate Professor, Professor, University of Chicago, 1946-61 |
| Professor, Stanford University, 1962-86 |
| Professor Emeritus, 1986 |
| Chairman, Department of Chemistry, University of Chicago, 1956-59 |
| Chairman, Department of Chemistry, Stanford University, 1972-74 & 1978-79 |
| Honors and Awards |
| Guggenheim Fellow, 1949 and 1955 |
| American Chemical Society Award for Nuclear Applications in Chemistry, 1955 |
| Harrison Howe Award, Rochester Section, ACS, 1960 |
| Chandler Medal, Columbia University, 1964 |
| John Gamble Kirkwood Award, New Haven Section, ACS, 1966 |
| ACS Award for Distinguished Service in the Advancement of Inorganic Chemistry, 1967 |
| Nichols Medal, New York, ACS, 1971 |
| Willard Gibbs Medal, Chicago Section, ACS, 1971 |
| F.P. Dwyer Medal, University of New South Wales, Australia, 1973 |
| Honorary Doctorate (L.L.D.) University of Saskatchewan, 1973 |
| Marguerite Blake Wilbur Endowed Professorship, 1976 |
| National Medal of Science, Washington, D.C., 1977 |
| Allied Chemical Award for Excellence in Graduate Teaching & Innovative Science, 1979 |
| Degree of Ph. D. Honoris Causa of the Hebrew University of Jerusalem, 1979 |
| T.W. Richards Medal of the Northeastern Section, ACS, 1980 |
| ACS Award in Inorganic Chemistry of the Monsanto Company, 1981 |
| The Linus Pauling Award, Puget Sound Section, ACS, 1981 |
| National Academy of Sciences Award in Chemical Sciences, 1983 |
| Bailar Medal, University of Illinois, 1983 |
| Doctor of Science, University of Chicago, 1983 |
| Robert A. Welch Foundation Award in Chemistry, 1983 |
| Nobel Prize in Chemistry, 1983 |
| Doctor of Science, Polytechnic Institute, New York, 1984 |
| Honorary Member, College of Chemists of Catalonia and Beleares, 1984 |
| Priestley Medal, ACS, 1985 |
| Doctor of Science, State University of New York, 1985 |
| Corresponding Member, Academy of Arts and Science of Puerto Rico, 1985 |
| Honorary Member, Canadian Society for Chemistry, 1986 |
| Distinguished Achievement Award, International Precious Metals Institute, 1986 |
| The Oesper Award, The Cincinnati Section of the American Chemical Society, 1986 |
| Doctor of Science, University of Guelph, 1987 |
| Honorary Member, Hungarian Academy of Sciences, 1988 |
| Doctor of Science, honoris causa, Seton Hall University, 1988 |
| Doctor of Science, Lajos Kossuth University of Debrecen, Hungary, 1988 |
| Honorary Fellowship, Royal Society of Chemistry, 1989 |
| Honorary Fellowship, Indian Chemical Society 1989 |
| G. M. Kosolapoff Award, Auburn Section, ACS, 1990 |
| Doctor of Science, Northwestern University, 1990 |
| Membership in Societies |
| American Chemical Society |
| National Academy of Sciences |
| American Academy of Arts & Sciences |
| Phi Beta Kappa |
| Sigma Xi |
| Phi Lamda Upsilon (honorary member) |
| Royal Physiographical Society of Lund |
| American Philosophical Society |
| Royal Danish Academy of Sciences & Letters |
| Foreign Member of the Finnish Academy of Science & Letters |
| Foreign Member, Royal Society |
| Corresponding Member, Brazilian Academy of Sciences |
| Foreign Associate, Engineering Academy of Japan |
| Corresponding Member, Australian Academy of Science |
| Consultantship |
| Catalytica Associates, Inc., Mountain View, California |
| Research Interests |
| Current research interests include: charge transfer as affecting properties including the reactivity of ligands; mixed valence molecules; mechanisms of “atom” and electron transfer reactions; basic chemistry of osmium and ruthenium, effects arising from back-bonding. |
| Publications |
| Over 350 scientific articles and a book have been published as a result of this research. |
Henry Taube died on 16 November 2005.
Henry Taube – Nobel Lecture
Nobel Lecture, December 8, 1983
Electron Transfer between Metal Complexes – Retrospective
Read the Nobel Lecture
Pdf 812 kB
Henry Taube – Facts
Press release

19 October 1983
The Royal Swedish Academy of Sciences has decided to award the 1983 Nobel Prize for chemistry to
Professor Henry Taube, Stanford University, Stanford, USA,
for his work on the mechanisms of electron transfer reactions, especially in metal complexes.
Chemistry prize awarded to one of the most creative contemporary workers in inorganic chemistry
Chemical reactions were known to man long before chemistry had attained the status of science. It was observed that substances changed their properties under certain external conditions, which is a characteristic of chemical reactions. Thus the ancient Egyptians found that if malachite, a green ore, was fired with charcoal, a red metal was obtained, called copper. It was also found that when clay was baked, ceramic products with properties quite different from clay were obtained.
Much earlier than this, man had found that a piece of dry wood caught fire if it could be made hot enough: changes in the properties of substances occurred only on certain conditions. Temperature was early the factor which was varied in order to bring about changes, and it was also found at an early stage that the speed with which the changes occurred frequently depended on the temperature. With the discovery of black powder it was also noted that processes could take place very rapidly, leading to explosions. The branch of chemistry concerned with how fast chemical reactions take place is known as chemical kinetics, and the scientist engaged in explaining how is said to study the mechanism of chemical reactions.
Millennia of hypotheses, experiments and observations, new hypotheses and new experiments and observations were to pass before a fairly firm scientific structure had been created. At the beginning of this century, progress had been considerable. In particular, a physical-mathematical description of the reactions had been produced, and it was possible in figures and formulas to express the conditions determining whether a chemical reaction would occur, and it was possible to provide mathematical equations for how rapidly it took place. A beginning had also been made in the treatment of reactions which did not pass completely in one direction, as opposed to those mentioned above. It was realized that chemical equilibria existed, and it was possible to deal with these theoretically. It is a characteristic of chemical equilibria that the reacting ions or molecules, although on average bound to another a given bond is not permanent and that the bonds are always being broken down and restored. Three major types of equilibrium reactions have come to be of dominant importance in chemistry. The concepts of acid and base were combined in the acid/base reactions and the pH associated with this.
Metal ions dissolved in water may attract ions or molecules. This is known as complex formation and usually, although not always, occurs as an equilibrium reaction. Finally the combustion of the burning piece of wood and the production of metallic copper from its ore through a reaction with charcoal have been generalized as oxidation and reduction. As a further generalization it has been found that oxidation and reduction are associated with a transfer of electrons, e.g. in metal ions such as cobalt and chromium. Under certain conditions it is possible to make cobalt with three positive charges react with chromium having two positive charges, where cobalt gets only two but chromium three positive charges. The effect is thus that an electron having a negative charge has been transferred from the two-valent chromium to the three-valent cobalt. This is particularly frequent phenomenon in complex compounds of metal ions. Taube has today been awarded the 1983 Nobel Prize for his studies of the mechanisms of electron transfer in metal complexes. Better than anyone else he has helped us understand how these electron transfers take place. It is particularly the structural preconditions governing electron transfers in metal complexes which he has studied. The electron transfer process as such is a separate major problem in theoretical chemistry and physics, where other scientists have contributed more than Taube.
What are the experiments made by Henry Taube and what conclusions has he been able to draw? In his studies, he started from the fact that three-valent ions of cobalt and chromium do not form equilibrium complexes (an example of the exceptions already referred to). The ions or molecules which are bound to these metal ions are therefore joined to them without ever leaving them. But the corresponding two-valent ions form equilibrium complexes. If an ion or molecule bound to the three-valent ion (in this instance, three-valent cobalt) could somehow be marked so that it is possible to find experimentally whether this marked ion or molecule in the electron transfer has at the same time been transferred to the other metal ion (in this instance, two-valent chromium), that is, in the opposite direction as the electron in this case. This was exactly what Taube found, and from this he drew the conclusion that before the electron transfer could take place, a bridge was formed between the metal ions of the ion or molecule which changed places. He proved this in a large number of cases and investigated how the electron transfer was affected by changes in the bridging molecule.
His next step was to lengthen the bridge between the metal ions (while using molecules which could bind two metal ions) and he found that in some instances there was still an electron transfer in spite of the greater distance between the metal ions. There was thus a form of what Taube calls ”distant attack”.
A logical continuation was the bonding of three-valent ions to the two ends of the bridge before reducing this complex with a two-valent ion (in this instance, europium). This reacted rapidly with one of the metal ions and Taube could then follow the slow transfer within the complex (in this case from ruthenium to cobalt) free from all assumptions on how rapidly the bridge was formed.
Finally Taube let the three-valent metal ions on either side of the bridge be identical and could then study if in reduction with an electron this was captured by one of the identical metal ions or it belonged to both, a phenomenon known as delocalization. (Delocalization generally gives rise to strong colours, such as in Prussian blue.)
This entire development was dominated both experimentally and theoretically by Taube, who according to one of the nominations has in eighteen listed instances been first with major discoveries in the entire field of chemistry. The examples selected here, which are all included in the prize award, may seem rather specialized, not to say esoteric. However, during the last ten years it has become increasingly apparent that Taube’s ideas have a considerable applicability, particularly in biochemistry. All respiration which is associated with oxygen consumption is thus also associated with electron transfers, and a growing number of scientists in this field are basing their work on Taube’s concepts of electron transfers in metal complexes.
It should be added that, as already pointed out, Taube has made major contributions throughout the chemistry of complexes. Thus he was the first to produce a complex between a three-valent metal ion, which was based on the ideas developed by Taube in his electron transfer studies.
Finally a quotation from one of Nobel Committee’s reports on Taube: ” There is no doubt that Henry Taube is one of the most creative research workers of our age in the field of coordination chemistry throughout its extent. He has for thirty years been at the leading edge of research in several fields and has had a decisive influence on developments.”
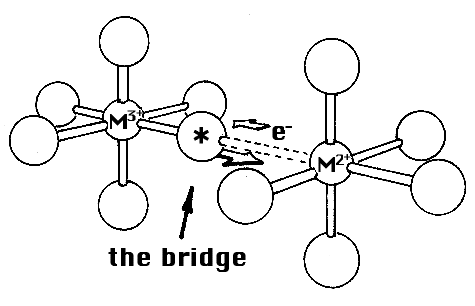
Figure: the bridge.
Award ceremony speech
Presentation Speech by Professor Ingvar Lindqvist of the Royal Academy of Sciences
Translation from the Swedish text
You Majesties, Your Royal Highnesses, Ladies and Gentlemen,
Henry Taube has been awarded the 1983 Nobel prize in chemistry for his studies of the mechanisms of electron transfer-reactions, particularly of metal complexes. I will not, during these few minutes, try to give a survey of the rich scientific production of Taube. Instead, I will choose one chemical reaction and attempt to show how our way of looking at this and similar reactions has changed drastically thanks to Taube.
Chemistry is a science which is rapidly aging. Most of the chemical investigations, basic or applied, which were published fifty years ago are to-day forgotten, not because they were wrong, but because our outlook of the science chemistry has changed so much during this time. Some few contributions are, however, still alive because they have determined in a decisive way our ideas about the fundamental relations in chemistry.
It must be in the spirit of Alfred Nobel that works of this type should be awarded the prize, each in its time. It can then be remembered that these new ways of thinking are in the long run also of utmost importance for applied chemistry, although it might be difficult to point directly to their usefulness at the occasion when the prize is awarded. Starting with Svante Arrhenius, who got the Nobel prize 1903, I will show how Taube fits in with this line of “new thinkers”. Arrhenius got the prize because he convinced his contemporary chemists that salts in aqueous solutions exist as positive and negative ions and not as neutral molecules. The reaction which I will discuss could, according to Arrhenius, be described as follows: Trivalent cobalt ions oxidize bivalent chromium ions and thereby form bivalent cobolt ions and trivalent chromium ions. The net result is an electron transfer between two positively charged ions. Arrhenius did not speculate further in molecular terms about the nature of the ions in the solution.
It was another Nobel prize winner (1913), Alfred Werner, who carried the development further in a decisive way when he proved that metal ions in solution are in many cases surrounded by a fixed number of neighbouring negative ions or neutral molecules. He also suggested that these neighbours are arranged in a certain way, e.g., in the corners of an octahedron if they are six in number. If Werner had known what we know to-day thanks to Taube, about the reaction I am discussing, he would have expressed the conditions in the following way: A trivalent cobolt ion surrounded by live ammonia molecules and one chloride ion is reacting with a bivalent chromium ion which probably is surrounded by six water molecules. During the reaction there are formed a bivalent cobalt ion surrounded by five ammonia molecules and one water molecule and a trivalent chromium ion surrounded by live water molecules and one chloride ion. The electron transfer is thus connected with a chloride ion transfer from cobolt to chromium. This description is certainly much more complete and gives a clear picture of the conditions before and after the reaction. It does not tell anything, however, about how the reaction has taken place.
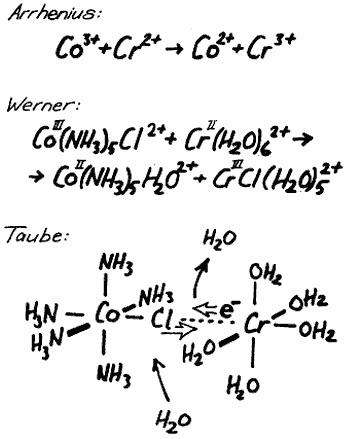
Taube has now proceeded one step further and has shown us exactly how the reaction occurs. The first step is the formation of a larger complex where the chromium ion is attached to the cobolt ion via the chloride ion which thus functions as a bridge between the two metal ions. This requires that a water molecule leaves the chromium ion to give place for the bridging chloride ion. The chromium ion will thus be surrounded by live water molecules and the bridging chloride ion. It is only when this bridge has formed that the electron transfer can take place making the cobalt ion bivalent and the chromium ion trivalent. Finally the bridge is opened and the chloride ion follows the trivalent chromium ion while the cobolt ion must take up a molecule of water to replace the chloride ion. In an impressive series of investigations Taube has developed and refined this idea and it will in the future be a natural part of the set of paradigms of chemistry, which thus in a decisive way has been enriched by the contributions of Henry Taube.
Professor Henry Taube,
I have not tried, during these few minutes, to give a comprehensive presentation of all your outstanding contributions to inorganic chemistry, but have rather preferred to try to show the conceptual importance of one of your achievements, which is an essential part of that work for which you have been awarded the Nobel prize. It is an honour and a pleasure for me to extend to you the congratulations of the Royal Academy of Sciences and to ask you to receive your prize from the hands of His Majesty the King.