Bruce Merrifield – Nobel Lecture
Nobel Lecture, December 8, 1984
Solid Phase Synthesis
Read the Nobel Lecture
Pdf 619 kB
Bruce Merrifield – Banquet speech
Bruce Merrifield’s speech at the Nobel Banquet, December 10, 1984
Your Majesties, Your Royal Highnesses, Colleagues, Ladies and Gentlemen,
I am very proud to be here today to experience this marvellous occasion. The hospitality and friendship of the Swedish people will not soon be forgotten.
I have asked myself why am I here – how did it happen – and the answer is difficult. I recalled my first day in James A. Garfield high school some 50 years ago and remembered the words of our 20th president that we were all required to memorize and recite. He said, in part, that if one had “a clear head, a true heart and a strong arm” he could succeed in life. I did not have all those qualities, but took him to mean that if you were honest and worked hard you could accomplish your goals. I have tried to follow his advice, but I never dreamed that it would lead me to this place where I stand today. Clearly there was more; it required good teachers, a good place to work, good friends, a good family and a great amount of good luck.
When the field of peptide chemistry was founded in 1901 by the great Emil Fischer he could not have imagined how it was destined to expand in so many important directions; into biochemistry, molecular biology, virology and nucleic acid chemistry. Because of that lucky and timely circumstance my contributions to the synthesis of this class of compounds have been amplified beyond my expectations. As so often happens, the idea behind the work was not fully accepted at the beginning, although some people were enthusiastic. I believe Emil Fischer would have been a supporter, however, and I am certain he would be happy to know that his far sighted vision of a totally synthetic protein was not just a dream.
On behalf of my many colleagues I want to express our gratitude for your recognition of our field.
Bruce Merrifield – Photo gallery
1 (of 1) Princess Christina of Sweden and Robert Bruce Merrifield at the Nobel Banquet in the Stockholm City Hall, Sweden, on 10 December 1984.
© Svensk Reportagetjänst 1984 Photo: Boo Jonsson
Bruce Merrifield – Other resources
Links to other sites
On Bruce Merrifield from Rockefeller University
On Bruce Merrifield from the American Society for Biochemistry and Molecular Biology
Speed read: Supporting protein chains
Creating the proteins that perform the host of tasks necessary to support life is not unlike creating a chain, link by link. In this case, the links, or amino acids, are attached sequentially to the growing chain, or peptide. Once the peptide is made, the chain folds up, either on its own or with others, to form a three-dimensional protein that can carry out its particular task.
What takes minutes or hours to achieve in Nature took months and years to recreate in the laboratory. Attaching amino acids together to form even small peptide chains was a laborious procedure, with chemists faced with the painstaking task of having to continuously fish out the fragile peptide chain from solution before adding each link. Bruce Merrifield’s solution for building chains of peptides in any predetermined order was simple, ingenious and radical. He proposed a method in which a molecular anchor provides the foundations for peptide synthesis. By attaching what would be the last amino acid in the peptide to a solid support made from a polymer, amino acids can then be chemically linked one by one in the correct order to generate the growing peptide chain. Once the construction process is completed the peptide can be released from the support.
The elegance of this method is that any by-products and unused starting ingredients that are not attached to the anchored peptide can be easily washed away after each step in the process, increasing yields to staggering levels. Within a few years, Merrifield had automated the peptide construction process, but his method wasn’t accepted until he showed it could create fully functioning proteins – from the 9-amino-acid-long peptide hormone bradykinin to his milestone synthesis of an active enzyme, ribonuclease A, created by attaching each of its 124 amino acids individually. His method was later adopted to create designer nucleotides, short fragments of specific sequences of DNA, which meant that Merrifield’s breakthroughs in chemistry evolved into an essential tool for molecular biology and biotechnology.
The Nobel Prize in Chemistry 1984
Bruce Merrifield – Biographical

Bruce Merrifield was born in Fort Worth, Texas, July 15, 1921, the only son of George E. and Lorene (Lucas) Merrifield. In the spring of 1923 they drove across the southwest desert to settle in California where they lived in several cities throughout the state. He attended nine grade schools and two high schools before graduating from Montebello High School in 1939. His interest in chemistry began there and he also enjoyed the astronomy club where he ground a mirror and built a small reflecting telescope. As a senior he managed to be runner up in the annual science contest and in the process learned a valuable lesson in the scientific method.
College began at Pasadena Junior College and at the end of two years he transferred to the University of California at Los Angeles (UCLA). After graduation in chemistry he worked for a year at the Philip R. Park Research Foundation taking care of an animal colony and assisting with growth experiments on synthetic amino acid diets. One of these was the experiment by Geiger that first demonstrated that the essential amino acids must be present simultaneously for growth to occur.
It soon became clear that more education was necessary and he returned to graduate school at the UCLA chemistry department with professor of biochemistry M.S. Dunn to develop microbiological methods for the quantitation of the pyrimidines. Graduation was on June 19, 1949, on June 20 he and Elizabeth Furlong were married, and on June 21 they left California for New York City and the Rockefeller Institute for Medical Research.
At the Institute, later Rockefeller University, he worked as an Assistant for Dr. D.W. Woolley who was to have a profound influence on his career. They worked on a dinucleotide growth factor he discovered in graduate school and on peptide growth factors that Woolley had discovered earlier. These studies led to the need for peptide synthesis and, eventually, to the idea for solid phase peptide synthesis in 1959. The development and application of the technique have occupied him and his laboratory up to the present date. He is very proud of the fact that his office was once occupied by the great pioneer peptide chemist, Max Bergmann, and has been inspired by the knowledge that his laboratories were once filled with names like Leonidas Zervas, Joseph Fruton, Klaus Hoffmann, Emil Smith, William Stein, and Stanford Moore.
In the meantime his wife, Libby, who was a biologist by training, stayed home in Cresskill, New Jersey, and raised their six children who now range in age from 19 to 32 years. They have been the great joy in the life of their parents; and now Jim has a daughter, Kelly, who is the pride of the whole family. Three years ago Libby joined the Merrifield laboratory at Rockefeller University.
He was a Nobel Guest Professor at Uppsala University in 1968 and was elected a member of the U.S. National Academy of Sciences in 1972. He has received several awards for his work on peptide chemistry including the Lasker Award for Basic Medical Research (1969), the Gairdner Award (1970), the Intra-Science Award (1970), the American Chemical Society Award for Creative Work in Synthetic Organic Chemistry (1972), the Nichols Medal (1973), the Instrument Specialties Company Award of the University of Nebraska (1977), and the 2nd Alan E. Pierce Award of the American Peptide Symposium (1979).
He has received honorary degrees from the University of Colorado (1969), Uppsala University (1970), Yale University (1971), Newark College of Engineering (1972), the Medical College of Ohio (1972), Colgate University (1977), and Boston College (1984). In 1984 he was appointed the John D. Rockefeller Jr. Professor of the Rockefeller University.
This autobiography/biography was written at the time of the award and later published in the book series Les Prix Nobel/ Nobel Lectures/The Nobel Prizes. The information is sometimes updated with an addendum submitted by the Laureate.
For more updated biographical information, see:
Merrifield, Bruce, Life During a Golden Age of Peptide Chemistry – The Concept and Development of Solid-Phase Peptide Synthesis. Oxford University Press, Oxford, 2001.
Bruce Merrifield died on May 14, 2006.
Bruce Merrifield – Facts
Press release

17 October 1984
The Royal Swedish Academy of Sciences has decided to award the 1984 Nobel Prize in chemistry to
Professor R. Bruce Merrifield, Rockefeller University, New York, USA,
for his development of methodology for chemical synthesis on a solid matrix.
Summary
R. Bruce Merrifield, Professor at Rockefeller University, has been awarded the Nobel Prize in chemistry for 1984 for his development of a simple and ingenious method for obtaining peptides and proteins. This method has created completely new possibilities in the field of peptide and protein chemistry, which is Merrifield’s own area of research, as well as in the field of nucleic acid chemistry, where other researchers have applied Merrifield’s ideas. Merrifield’s method has greatly stimulated progress in biochemistry, molecular biology, pharmacology and medicine. It is also of practical importance, both for the development of new drugs and for gene technology. Merrifield’s method involves binding the first of the many amino acid residues of which a protein is composed to a special substance called a polymer. Such a synthesis is more rapid than those achieved with earlier methods and the quantity of final product obtained is considerably greater.
Background information
The chemical reactions which take place in living organisms are not spontaneous, but require the involvement of catalysts. These catalysts are called proteins. The first step in the formation (biosynthesis) of a protein in a living organism is the construction of a linear molecule called a peptide and composed of a large number of amino acid residues. In general these peptides can be subsequently modified in a number of ways. There are about 20 amino acids which are commonly used in the biosynthesis of proteins and the number of possible variations is virtually unlimited. A number of hormones and other signal substances which regulate different life processes are also peptides, but these compounds contain considerably fewer amino acid residues than do proteins.
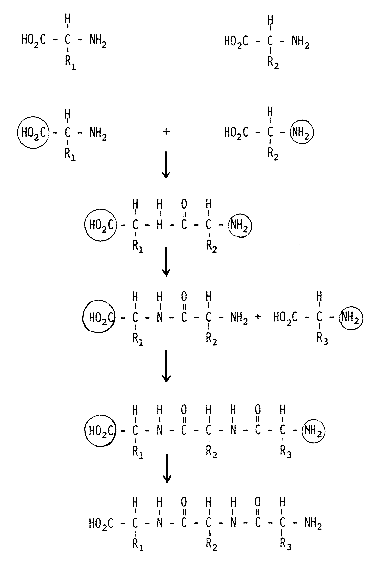
We know the detailed structure of a large number of proteins and peptides, thanks to the efforts of Frederick Sanger (Nobel Prizewinner in 1958) and Stanford Moore and William H. Stein (Nobel Prizewinners in 1972), among others. A very important contribution was also made by the Swedish researcher Pehr Edman, who unfortunately died relatively young but who left behind an automated method for determining peptide structure which is now used routinely throughout the scientific world. The chemical synthesis of peptides is an important task for organic chemists and the principle generally used today for such synthesis was developed a relatively long time ago by the Nobel Prizewinner in chemistry for 1902, Emil Fischer (Fig.1). All amino acids contain two typical functional groups, an acid (carboxylic) group and a basic (amino) group. When the amino group in one amino acid reacts with the carboxylic group in another, the resulting chemical bond gives rise to a dipeptide. In order for this reaction to take place in a controlled manner, the carboxylic group on amino acid 1 and the amino group in amino acid 2, groups which are not to be involved in the reaction, must be protected. If one of these protecting groups in the dipeptide is later selectively removed and reaction with a third amino acid containing one protected group carried out, a tripeptide is formed. And so forth. This approach is simple in theory, but difficult in practice. After each step the product must be separated from biproducts and unreacted starting material and loss of product is inevitable during such separation. When the Nobel Prizewinner in chemistry for 1955, Vincent du Vigneaud, synthesized the peptide hormone oxytocin, which is a nonpeptide, this was a very great step forward. To use this method for synthesizing peptides containing some 100 amino acid residues is truly an heroic task. If the yield in each synthetic step is 90%, which is a very good yield, then the overall yield after 100 steps would be 0.003%. In order to obtain measurable amounts of the final product the first steps of the synthesis would thus have to be conducted on a very large scale and the synthesis becomes very tedious.
Merrifield solved this problem in a manner which is both simple and ingenious (Fig. 2). He attaches the first amino acid through its carboxylic group to a solid polymer. After each synthetic step, byproducts and remaining starting materials can thus be removed by filtration and washing the polymer. Only when the entire synthesis has been completed is the peptide removed from the polymer. The advantages of this method are very considerable. Through the replacement of a complicated isolation procedure for each intermediate product with a simple washing procedure much time is saved. In addition, it has proven possible to increase the yield in each individual step to 99.5% or better, a result which cannot be attained using conventional synthetic approaches. In the example given above the final overall yield would thus be increased from 0.003% to 61%. Finally, this method is also suitable for automation and automatic peptide synthesizers are now commercially available.
Merrifield’s methodology has brought about a revolution in peptide and protein chemistry and thousands of different peptides have now been synthesized using this approach. One milestone in this respect was the synthesis of an active enzyme, ribonuclease A, by Merrifield himself and his coworkers.
Merrifield’s idea of performing a multistep synthesis with a compound attached to a solid matrix as the starting material has also been used in other areas. The most important of these is undoubtedly the synthesis of oligonucleotides, which are needed in hybrid DNA research. In this case as well an automated apparatus which can be programmed to synthesize desired products has been constructed. Although Merrifield has not worked in this area himself, it is clearly his ideas which have found a new application here.
Merrifield’s methodology is a completely new approach to organic synthesis. It has created new possibilities in the research fields of peptide-protein chemistry and nucleic acid chemistry It has greatly stimulated progress in biochemistry, molecular biology, pharmacology and medicine. It is also of great practical importance, both for the development of new drugs and for gene technology.
Figure 1. Synthesis of a tripeptide using conventional methodology. HO2C- is a carboxylic group, NH2- an amino group. A circle around a group indicates that it is protected and, thus, cannot react.
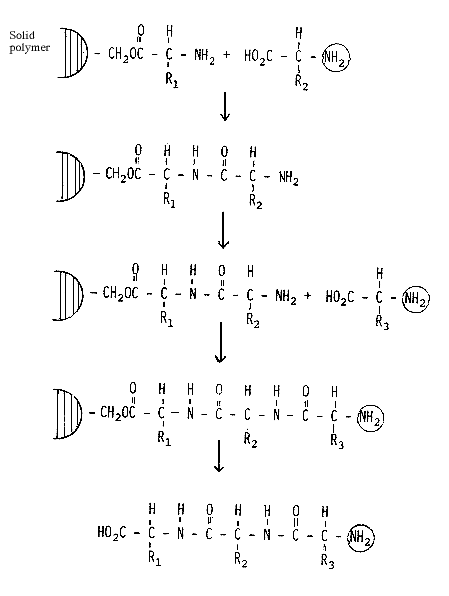 |
Figure 2. Synthesis of a tripeptide using Merrifield´s methology.
Award ceremony speech
Presentation Speech by Professor Bengt Lindberg of the Royal Academy of Sciences
Translation from the Swedish text
Your Majesties, Your Royal Highnesses, Ladies and Gentlemen,
The chemical reactions which take place in living organisms are not spontaneous, but require the involvement of catalysts. These catalysts are called proteins and are composed of chains of amino acids called peptides. A number of hormones and other substances which regulate different life processes are also peptides. There are about 20 naturally occurring amino acids which are found in such peptides and since the chains can be very long, the number of possible variations is virtually unlimited.
Today we know the structures of a very large number of proteins and peptides. Important contributions to this area of knowledge were made by Fredrick Sanger, who received the Nobel prize in 1958, and Stanford Moore and William H. Stein, Nobel prizewinners in 1972. A very important contribution was also made by the Swedish researcher Per Edman, who unfortunately died relatively young and whose method for the controlled degradation of peptides is now generally used.
The chemical synthesis of peptides is an important task. The principle used in such synthesis is simple and was developed a relatively long time ago by Emil Fischer, who received a Nobel prize in 1902, although for completely different discoveries. Expressed simply, this principle involves the binding together of two amino acids which have been appropriately modified to give a dipeptide. This dipeptide is then combined with a third modified amino acid to give a tripeptide and so on.
Even if the principle is simple, in practice it is difficult to synthesize peptides, since a large number of individual steps is involved. After each step the desired product must be separated from by-products and unreacted starting material and this takes time and involves loss of the product. When Vincent du Vigneaud synthesized a peptide hormone, oxytocin, which is a nonapeptide, for the first time, this represented a great step forward which was rewarded with the Nobel prize for 1955. To use a similar approach for synthesizing a peptide containing 100 or more amino acid residues is truly a heroic task, requiring a very large amount of work and chemicals. This task can be compared to climbing a high mountain peak in the Himalayas, which begins with a large expedition carrying much equipment and ends, if all goes well, with a few lightly equipped alpinists reaching the top.
Therefore, Merrifield’s development during the 1960’s of a method for carrying out peptide synthesis on a solid matrix revolutionized the field. He attached the first amino acid to an insoluble polymer, a plastic material in the form of small spheres. Subsequently, the other amino acids were added one after one and only after the entire peptide chain had been synthesized was it released from the polymer. The advantages of this method are considerable. The complicated purification of the product after each synthetic step is replaced by simply washing the polymer to which the peptide is attached, so that loss of product is avoided completely. At the same time, the yield for each individual step is increased to 99.5% or better, a goal which cannot be achieved with conventional methods, but which is extremely important in syntheses involving a large number of steps. Finally, this method can be automated and automatic peptide synthesizers are now commercially available.
Thousands of different peptides of different sizes, as well as proteins, peptide hormones and analogues of these compounds have now been synthesized using this method. One milestone in this respect was the synthesis of an active enzyme, ribonuclease, containing 124 amino acid residues, by Merrifield and his coworkers.
The approach of performing a multistep synthesis with a compound attached to a solid matrix as the starting material has also been used in other areas. The most important of these is undoubtedly the synthesis of oligonucleotides, which are needed in hybrid DNA research. In this case as well an automated apparatus which can be programmed to synthesize desired products has been constructed. Although Merrifield has not worked in this area himself, it is clearly his ideas which have found a new application here.
Professor Merrifield,
Your methodology for chemical synthesis on a solid matrix is a completely new approach to organic synthesis. It has created new possibilities in the fields of peptide-protein and nucleic acid chemistry. It has greatly stimulated progress in biochemistry, molecular biology, medicine and pharmacology. It is also of great practical importance, both for the development of new drugs and for gene technology.
On behalf of the Royal Swedish Academy of Sciences I wish to convey our warmest congratulations and ask you to receive your prize from the hands of His Majesty the King.