The Nobel Prize in Chemistry 1990
Elias James Corey – Nobel Lecture
Nobel Lecture, December 8, 1990
The Logic of Chemical Synthesis: Multistep Synthesis of Complex Carbogenic Molecules
Read the Nobel Lecture
Pdf 761 kB
Elias James Corey – Banquet speech
Elias J. Corey’s speech at the Nobel Banquet, December 10, 1990
Your Majesties, Your Royal Highnesses, Ladies and Gentlemen,
There is a children’s poem by Julia Carney entitled “Little Things,” one verse of which reads:
Little drops of water, little grains of sand
Make the mighty ocean and the pleasant land
Thus the little minutes, humble though they be
Make the mighty ages of eternity.
It is by countless small steps, like the little grains of sand, that we endeavor to understand ourselves and our universe, though confronted by endless complexity. Whatever our field – science, technology, humanities, art – we are excited by the challenge of research at an endless frontier, and also humbled by the vastness of our ignorance. We are heartened by the belief that knowledge, if wisely used, can benefit mankind.
My special fascination has been to understand better the world of chemistry and its complexities. Chemical compounds of carbon can exist in an infinite variety of compositions, forms and sizes. The naturally occurring organic substances are the basis of all life on earth, and their science at the molecular level defines a fundamental language of that life. The chemical synthesis of these naturally occurring substances and many millions of other carbon compounds has been one of the major enterprises of science in this century. That fact was affirmed by the award to me of the Nobel Prize in Chemistry for 1990 for the “development of the theory and methodology of organic synthesis.” Chemical synthesis is uniquely positioned at the heart of chemistry, the central science. Its impact on our lives and society is all pervasive. For instance, most of today’s medicines are synthetic and the majority of tomorrow’s will be conceived and produced by synthetic chemists. To the field of synthetic chemistry belongs an array of responsibilities which are crucial for the future of mankind, not only with regard to the health and needs of our society, but also for the attainment of a deep understanding of matter, chemical change and life.
In the years after World War II, chemical syntheses were developed which could not have been anticipated in the earlier part of this century. For the first time, several complex molecules were assembled by elaborately conceived multistep processes, for example vitamin A, cortisone, morphine, penicillin, and chlorophyll. This striking leap forward was followed more recently by an equally dramatic advance in which chemical synthesis has been raised to a much higher level of sophistication. Today, in many laboratories around the world, chemists are synthesizing at an astonishing rate complex organic structures which could not have been made in the 1950’s or 1960’s. This advance has been propelled by the availability of new ways of thinking about chemical synthesis and the use of new chemical and physical methods. Many talented investigators all over the world have contributed to the latest surge of chemical synthesis. Their efforts constitute a collective undertaking of vast dimensions, even though made independently. Their ideas and discoveries interact synergistically to the benefit of all. I am happy to have been selected by the Nobel Committee for contributions to the science of chemical synthesis, but I am even more pleased that this important field of science has again received high recognition.
Award ceremony speech
Presentation Speech by Professor Salo Gronowitz of the Royal Swedish Academy of Sciences
Translation from the Swedish text
Your Majesties, Your Royal Highnesses, Ladies and Gentlemen,
Organic synthesis – the preparation of complicated organic compounds, using simple and cheap starting materials – is one of the prerequisites of our civilization, the chemical age in which we live. As late as the 1820s it was still believed that organic natural products such as sugar, camphor or morphine were endowed with a special vital force and therefore could not be prepared in the laboratory. In 1828, the German chemist Friedrich Wöhler crushed this dogma by preparing the organic compound urea from inorganic ammonium cyanate. Today, the most complicated natural products can be prepared, which in their three-dimensional structure are completely identical with those isolated from nature. This year’s Nobel Laureate has made extremely important contributions in this area.
In spite of these developments, the delusion still thrives in many quarters that there are some special advantages in the natural product over the synthetically prepared one. However, Vitamin C is Vitamin C, regardless of whether it is isolated from citrus fruits or synthesized in chemical factories.
The development of organic synthesis during a period of a little over one hundred years has afforded efficient industrial methods for the preparation of paints and dyes, pharmaceuticals and vitamins, insecticides and herbicides, which increase our harvests, plastics and textile fibers, which clothe humanity. Organic synthesis has contributed to the high standards of living and health and the longevity enjoyed at least in the Western world. It is understandable that contributions to organic synthesis have often been rewarded with the Nobel Prize in Chemistry.
The synthesis of complicated organic compounds often shows elements of artistic creation. Many earlier syntheses were performed more or less intuitively, so that their planning was difficult to perceive. Asking a chemist why he chose precisely the starting materials and reactions that so elegantly led to the desired result would probably be as meaningless as asking Picasso why he painted as he did.
The process of synthetic planning has been compared to a game of three-dimensional chess using 40 pieces on each side. But the problem of synthesis may be even harder than this. Over 35,000 usable methods of synthesis are described in the chemical literature, each with its possibilities and its limitations. During synthesis, moreover, new methods appear which can modify the strategy. It is like allowing new moves during a game of chess.
Beginning in the 1960s Professor E.J. Corey coined the term and developed the concept of retrosynthetic analysis. Starting from the structure of the molecule he was to produce, the target molecule, he established rules for how it should be dissected into smaller parts, and what strategic bonds should be broken. In this way, less complicated building blocks were obtained, which could later be assembled in the process of synthesis. These building blocks were then analyzed in the same way until simple compounds had been reached, whose synthesis was already described in the literature or which were commercially available. Corey showed that strict logical retrosynthetic analysis was amenable to computer programming. He is the leader in this rapidly developing field.
Through his brilliant analysis of the theory of organic synthesis, Corey has been able to carry out total syntheses of around a hundred naturally occurring biologically active compounds, according to simple logical principles, which previously were very difficult to achieve. Only a few of his achievements in organic synthesis can be mentioned here. In 1978, he prepared gibberellic acid, which belongs to a class of very important plant hormones of complicated structure. Corey has furthermore synthesized gingkolid B, which is the active substance in an extract from the ginkgo tree and is used as a folk medicine in China.
Corey’s most important syntheses are concerned with prostaglandins and related compounds. These often very instable compounds are responsible for multifarious and vital regulatory functions of significance in reproduction, blood coagulation and normal and pathological processes in the immune system. Their importance is witnessed by the awarding of the 1982 Nobel Prize in Physiology or Medicine to Professors Sune Bergström, Bengt Samuelsson and Sir John Vane for their discovery of prostaglandins and closely related biologically active compounds.
With enormous skill Corey has carried out the total syntheses of a large number of such compounds. It is thanks to Corey’s contributions that many of these important pharmaceuticals are commercially available.
To perform these total syntheses successfully, Corey was also obliged to develop some fifty entirely new or considerably improved synthesis reactions. His systematic use of different types of organometallic reagents has revolutionized recent techniques of synthesis in many respects. In recent years, he has also introduced a number of very effective enzyme – like catalysts, which yield only one mirror isomer of the target product in certain types of synthetically important reactions. No other chemist has developed such a comprehensive and varied assortment of methods, often showing the simplicity of genius, which have become commonplace in organic synthesis laboratories.
Corey has thus been rewarded with the Prize for three intimately connected contributions, which form a whole. It can be summarized in the following way. Through retrosynthetic analysis and introduction of new synthetic reactions, he has succeeded in preparing biologically important natural products, previously thought impossible to achieve. Corey’s contributions have turned the art of synthesis into a science.
Professor Corey,
In these few minutes I have tried to explain your immense impact on the theory and methodology of organic synthesis. In recognition of your important contribution to chemistry, the Royal Swedish Academy of Sciences has decided to confer upon you this years’ Nobel Prize for Chemistry. It is an honour and pleasure for me to extend to you the congratulations of the Royal Swedish Academy of Sciences and to ask you to receive your Prize from the hands of His Majesty the King.
Elias James Corey – Other resources
Links to other sites
Elias J. Corey’s page at Harvard University
MIT Digital Thesis Library – “The synthesis of N,N-diacylamino acids and analogs of penicillin” and
“The condensation of citraconic and maleic anhydrides with chloroprene and ethoxyprene” by Elias J. Corey
Elias James Corey – Photo gallery
1 (of 5) Elias James Corey receiving his prize from H.M. King Carl XVI Gustaf of Sweden at the Stockholm Concert Hall, on 10 December 1990.
Nobel Foundation. Photo: Lars Åström
2 (of 5) All 1990 Nobel Prize laureates assembled on stage at the Nobel Prize award ceremony in Stockholm, Sweden on 10 December 1990: physics laureates Jerome I. Friedman, Henry W. Kendall and Richard E. Taylor, Chemistry laureate Elias James Corey, medicine laureates Joseph E. Murray and E. Donnall Thomas, literature laureate Octavio Paz and economic sciences laureates Harry M. Markowitz, Merton H. Miller and William F. Sharpe.
Nobel Foundation. Photo: Lars Åström
3 (of 5) Elias James Corey talking to HM Queen Silvia of Sweden at the Nobel Banquet in the Stockholm City Hall, Sweden, on 10 December 1990.
© Svensk Reportagetjänst 1990 Photo: Boo Jonsson
4 (of 5) Elias James Corey and HM Queen Silvia of Sweden at the Nobel Prize banquet in the Stockholm City Hall, Sweden, on 10 December 1990.
Nobel Foundation. Photo: Lars Åström
5 (of 5) Elias James Corey photographed during Nobel Week in Stockholm, December 1990.
Nobel Foundation. Photo: Lars Åström
Speed read: Back to the future
The achievements awarded the 1990 Nobel Prize in Chemistry demonstrate how the secret to constructing synthetic versions of chemicals from scratch is to work backwards. Elias Corey developed and refined a novel, reverse approach to synthesizing organic compounds – that is, compounds that contain carbon atoms – and in the process he transformed this discipline from an art into a more logical and systematic process.
When Corey began his career in the 1950s, creating synthetic versions of organic compounds was carried out on a case-by-case basis, and relied largely on trial and error. Skilled exponents, such as Robert Woodward, who received the 1965 Nobel Prize in Chemistry, built compounds of a complexity unseen at the time by spotting possible starting subunits within the structure of a chemical, and devising ways in which to tweak and manipulate these subunits to generate the complete molecule. Corey, on the other hand, believed in a more rational approach that began with the theoretical structure of the end product. By strategically breaking key bonds within the complete molecule, he envisaged how it could be split into progressively simpler fragments until one is left with fragments that are already known and available in the laboratory, from which the building process could begin.
Corey formulated a set of general principles and guidelines, which he termed retrosynthetic analysis, to logically determine how best to break up any complex compound into simple precursor fragments and to work out the best path to constructing them from starting materials. Putting his own theories into practice, Corey devised routes for successfully synthesizing over 100 compounds; perhaps the most notable being his construction of synthetic versions of prostaglandins, which are involved in a host of important biological processes, including the regulation of blood pressure and the heart. When chemists were limited by the existing synthetic reactions that they had in their chemistry set to reconstruct fragments, Corey developed a series of new reactions that could force fragments together in different ways. Eventually computer programs were created using Corey’s principles and guidelines, allowing chemists to design new molecules and pinpoint the simplest possible paths for synthesizing them.
Elias James Corey – Facts
Credits and References for the 1990 Chemistry Nobel Poster
Editors:
Solgerd Björn-Rasmussen and Margareta Wiberg Roland, Information Department, The Royal Swedish Academy of Sciences
Author:
Dr Olof Wennerström, Department of Organic Chemistry, Chalmers University of Technology, Göteborg, Sweden
Illustrator:
Karin Feltzin, Stockholm, Sweden
Printed by:
Tryckindustri AB, Solna 1990
Copyright © The Royal Swedish Academy of Sciences, Information Department, Box 50005, S-104 05 Stockholm, Sweden,
Tel. +46-8-673 95 00, Fax +46-8-15 56 70, Email: info@kva.se
Web Adapted Version:
Nobelprize.org
Every effort has been made by the publisher to credit organizations and individuals with regard to the supply of photographs and illustrations. The publishers apologize for any omissions which will be corrected in future editions.
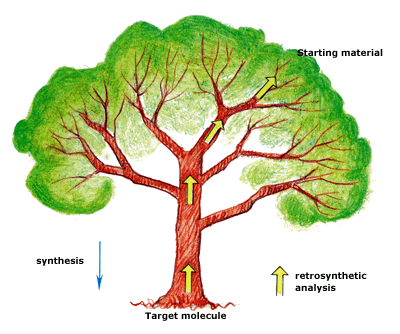
Retrosynthetic tree
Retrosynthetic analysis can be compared to the climbing of a tree. You are at the root of the tree (the target molecule) and want to climb to the top branches (the starting materials). The tree branches off many times. You have to choose your way! Which way is the fastest, the easiest and the most reliable to the best starting materials? E.J. Corey has developed and analysed many different strategies for making such a choice. One might e.g. choose to use a specific reaction. Alternatively, one might choose to build (synthesize) a particular part of the target molecule first or choose to solve some specific problem associated with the three-dimensional structure of the target, its stereochemistry.
Today, the analysis can be performed with computers in which an ever increasing number of reactions are stored. The computer gives suggestions, but the chemist must choose the way and reactions from his/her own qualifications and experience.