May-Britt Moser
Biographical

I was born and raised in Fosnavåg, a small town on an island on the west coast of Norway, in one of the most beautiful parts of the country (Fig. 1).
My parents owned a small farm, although my father worked as a carpenter. My mother took most of the responsibility for the farm and cared for me and my four older siblings, in addition to having small jobs now and then. Before I was born, we had a lot of animals on the farm, with cows, chickens and a horse, but by the time I was born, we only had sheep. Both my father and mother worked very hard all the time, and I learned at an early age that work makes you happy.

I was a happy, curious child with a lot of dreams and a lucky star above my head. I was also a tomboy who played with the boys a lot. But because we were five children, we didn’t have much money and we didn’t have a car, so I would stay home during the summers when my friends would go away. I was the youngest child by 10 years, and had a lot of time to myself to study animals on my own, and I loved it. I would spend time out in our fields where I would play by myself. I even studied the behaviour of snails as they ate grass. As I watched, I would always wonder about the reasons behind what the animal was doing.
My mother liked to tell me fairy tales, but she didn’t want to frighten me with the scary parts. Instead, she would tell me the parts of the stories that talked about hopes and dreams, like “Askeladden,” a boy who had nothing, but he used his head, he was kind and he worked hard – so he succeeded. I loved it when my mother would read me these fairy tales. It also made me believe that even though you have nothing you can become something – it was a bit of the American dream!
My mother had her own dreams, too: she wanted to be a doctor. The area where I grew up is somewhat religious, so I also met missionaries who had travelled to distant countries. My mother’s dream and the experience of meeting missionaries made me eager to go abroad so I could work as a doctor and save the world. I also loved animals, so I thought perhaps if I didn’t study to be a doctor, I could be a veterinarian. My dad had taught me to care for animals, and was a warm, good role model for me.
Schoolyears
I was not always the best student with the highest grades, but my teachers saw something in me and tried to encourage me. My mother had persuaded the local school officials to let me start school a year earlier than other students because my birthday was in January (the cut-off was the end of December) and because she saw that I was ready. My main primary school teacher would say “Wow, you are the youngest child in the class, and yet you still know this!”
I also had a teacher in high school who would call on me in class and say, “Frøken Andreassen, you know the answer! I believe in you!” And I had a physics teacher who really encouraged his female students by telling us that he wanted us to come back and show him that we had become engineers. There were other teachers, too, like my Norwegian teacher who said she thought my writing was good, but the bottom line is that I felt like I got a lot of special attention, and I was noticed. It made a difference.
At the same time, though, I wasn’t that motivated in high school, because I spent too much time with friends, and I didn’t have the drive to get the grades I needed to get into medical school. But my grades were still good, because my mother warned me that if I didn’t work hard I would have to go to school to study home economics and be a housewife. That thought horrified me.
The University of Oslo
When it came time to go to university, I decided I would go to the University of Oslo, in part because I had two older sisters in the Oslo area. I was also able to live for some time with one sister in Asker, an hour outside Oslo, while I looked for a place to live.
I loved university, it was fantastic – there was so much freedom, and I was very social. But I still wasn’t sure what I wanted to do. I loved mathematics and physics in high school, and thought about studying biology or geology and maybe becoming a teacher, but I didn’t see myself as a teacher. I also applied to dentistry school, in part because I had a boyfriend at the time who was studying dentistry. The dentistry school accepted me, but I decided not to pursue that either.
It was about that time that I met Edvard with a friend of mine on Karl Johans gate, the main pedestrian mall in downtown Oslo. I recognised him, of course, as the smart kid from my high school. He was visiting before he started at the university, and when I found out that he would be coming back to Oslo to start his studies in January, I told him to look me up so that I could show him around. And he did.
We quickly became friends. We decided that we would study psychology together, so we could learn about the brain. That brought me back to my dreams of my childhood, when I was so eager to understand why we do things. I was in heaven.
During our first year in the main psychology programme we were in the same social psychology class, called Psychology of Small Groups. We published a paper with several classmates and our teacher, Professor Skårdal, as a result of our research from that class. This was our first paper – “The interactional effects of personality and gender in small groups: A missing perspective in research,” published in the International Journal of Small Group Research. Professor Skårdal liked us so much he tried to encourage us to study social psychology, but we said, “No thanks, we want to study the brain!” We simply burned with eagerness to understand the brain.
Terje Sagvolden and Per Andersen
We started in Terje Sagvolden’s lab during our second semester. He was studying hyperactivity in rats. At the same time, our own relationship had changed from a friendship to a romance, and we got engaged on top of Mt. Kilimanjaro. It was a dream place for both of us – Edvard loves volcanoes, all volcanoes, and since my childhood encounters with missionaries, I had always wanted to go to Africa.
Working in the Sagvolden lab involved studying pure behaviour for two years. It was quite exciting, and it was interesting to work with rats, to try to understand why these animals were hyperactive. He taught us experimental design, especially the need to have controls in an experiment, and we learned a lot of behavioural theory. But we pushed him very hard, we kept asking him, “Can’t you go into the brain?” We had this crazy energy, this drive to know – it wasn’t just Edvard, or just me, it was the two of us together.
Eventually it came time for us to do our master’s thesis work and we realised that if we were going to have any opportunity to study the brain directly, we needed to work with Per Andersen in the neurosciences group. This was a problem, because we knew he didn’t really like psychologists – all the people in the Department of Neurosciences at that time were medical doctors – and that his research group was full.
When we finally got to talk to him, I decided that I was not going to leave the room until he accepted us as graduate students. I felt like I had been glued to the chair. In the end, I think he just realised he couldn’t get rid of us. He finally told us, “Fine, if you are going to do your master’s research here, you have to read this paper (by Richard Morris on water mazes), see if you understand it, and then build a water maze lab. If that is a success, then you will be allowed to do a master’s thesis in my lab.” I remember I said, “Oh wonderful, because we want to do a PhD with you, too!”
Building a Water Maze Lab
Per wanted us to build a water maze literally from scratch – a tank 2 metres in diameter by 50 cm high. When we left the meeting, I said to Edvard, “This is crazy!” Fortunately my brother-in-law worked at Det Norske Veritas and I called him, and he was able to help us buy a tank. We also had to get a marine pump so we could pump 1,250 litres of water out of the tank – we had a hose so we could pump the water into a toilet across the corridor. The water had to have milk in it because it had to be murky. That meant we had to change the water every day or it would smell, because the water temperature had to be at 25 degrees C so that the rats would feel comfortable in the pool when they searched for the hidden platform.
So we were psychology students during the day, and then worked in our lab at night. Per had a programmer who helped us write a programme so that we could track the rats as they swam in the maze – this was at the point where you couldn’t buy anything off the shelf. Early on we realized we had to use hooded rats rather than the albino Wistar rats, because it was easier to track their movement and because they are dark eyed and have better vision than the red-eyed albino rats.
Per showed us how to make tiny lesions in the hippocampus, because he had this idea that he wanted to study LTP in the living brain. Long-term potentiation, LTP, is how the connections between neurons in the brain are strengthened, and had actually been discovered in 1966 by Terje Lømo, in Per’s group and supervised by Per. We first had to make lesions in the dorsal and ventral parts of the hippocampus so that a hippocampal slice was left on both sides of the brain. Per’s idea was that it would be easier to detect LTP in the living brain if the area where such changes could occur was restricted. This was a brilliant idea, but in order to find out how big this slice had to be to support learning we had to make lesions of different sizes, both in the dorsal and the ventral hippocampus. Theodor Blackstad helped us to figure out where the boundaries of the hippocampus and the subiculum were.
The challenge for us was that we were psychologists, we didn’t know much about the brain’s anatomy. We first had done one brain dissection on a human cadaver, and that was it. But we learned fast.
Looking for LTP
Per’s idea was to give the animals extensive training in the water maze so after they had learned a lot, we could measure the changes in the tiny hippocampal slice that was left in the brain. He predicted that this slice would have increased synaptic efficiency (LTP) compared to animals who did not learn. He was so excited when he told us about his dream. His excitement was so important for us, and he was a great inspiration.
We thought that we first needed to find out if the animals could learn at all if they had these kinds of lesions, and then we also needed to have controls. We needed to know if we left the middle part of the hippocampus untouched, would the rats be able to learn if we removed the dorsal part, and what would happen if we removed the ventral part.
Once we did this, we found out that when the rats had a lesion in the dorsal part of the hippocampus they didn’t learn, but if they had a lesion in the ventral part they were fine – if we made large lesions in the ventral part they could still navigate perfectly well. The hippocampus was known to be involved in this behaviour, but what we found out was that it was only the dorsal part of the hippocampus that was involved in spatial learning and memory, and that the hippocampus was functionally heterogeneous even though it looked like a smooth sausage from the outside.
The Pink Poster
Per was president of the European Neuroscience Association (ENA) at that time, and so he allowed us to bring a poster of our research to a meeting of the ENA in Sweden. I have to confess that when I made the poster, I made it pink as a way to tease Per a bit. But maybe the pink also helped it to stand out, because when Richard Morris walked past the poster he commented that he thought our findings on the dorsal-ventral difference were interesting, and he also saw that we had used the water maze.
But he told us that he didn’t like our lesion method, because we had used aspiration, which can cut passing axons. He encouraged us to work with Len Jarrard who had just had a sabbatical in Edinburgh and who was an expert in making lesions without removing the fibres, and he thought that was crucial. Richard gave a plenary talk at the meeting and he mentioned our poster, and I thought that was pretty amazing. Edvard and I were so proud!
We published our results in The Journal of Neuroscience. This was also our joint master’s thesis – we were able to write it together. It was a pretty thick thesis, and a pretty thorough study of the two parts of the hippocampus. We got help from a few external people who came to the lab because Per was an internationally recognised scientist. These visitors included Eric Kandel from Columbia University, who told Per, “You really have to publish this with them,” and Larry Squire from the University of California, San Diego, who also encouraged Per to let us publish our results.
Funding for Two PhDs
Our experiment also raised the question, if the dorsal part is involved in memory, what does the ventral part do? So we started to read a lot about anatomy, and wondered about the nature of the connections between the entorhinal cortex and the dorsal and the ventral parts of the hippocampus. That was the first time we wrote to Menno Witter at the Free University in Amsterdam, because he had done a lot of work on hippocampal connections.
As we finished our master’s thesis, we both wanted to continue with Per on our PhDs, but the challenge was how to get two fellowships to study with him. At the time it was very difficult to get this kind of funding. The Research Council of Norway at the time was concerned about geographic distribution of these grants.
Per had one question that he felt certain would be funded, the relationship between long-term potentiation and memory, which was both a timely and very interesting question. Per told us that we would have to decide which one of us would take the topic and get funded for a PhD. I told Edvard that I thought Per’s question was a good topic for him, since he was so interested in the question. Then Per told me that he thought I should also get a PhD, and that he would help me find money.
His plan was for me to collaborate with a colleague of his, Jørg Mørland, in the Toxicology Department, to study what happened to hippocampal synapses if you gave alcohol to an animal. But I didn’t like the topic, because I thought the manipulations were too general to learn anything specific about learning and memory and also because I couldn’t see myself giving rats the high, high doses of alcohol that you would need to see an effect. So I found a way to convince him that I couldn’t do the project – I told him that I was from the Bible Belt of Norway and I simply can’t do this. He pushed me but I just refused.
But I was very interested in the fact that we could see synapses with a laserscanning confocal microscope. This was very new at the time – we had just got the microscope, and it was new enough that people didn’t believe that it was possible to see synapses with this equipment. But Per had a dream, and was enthusiastically convinced that it should be possible. Per was still trying to get me to do experiments with alcohol, but I said, no, I’m a psychologist, I want to do exactly the opposite – instead of trying to see if alcohol will reduce the number of synapses, I want to train the animals to see if there is an expansion of the number of synapses with learning. So I started to read, and I was convinced it was possible.
What was interesting was that Per was so sure that this project would be a failure and that it would not be funded that he tried to stop me from sending the grant proposal to the Research Council. I kept going to his office with the application I wrote to see if he had changed his mind, and finally he just gave in – he always had to give in, like my dad typically gave in when I insisted. Then I sent in my application, and both Edvard and I got grants – a surprising and joyful moment in our lives.
PhD Research
By then we had gotten married, on July 27, 1985 in Oslo. We had the same supervisor, we were studying the same structure in the brain, but we still both got funded from the Research Council of Norway. It was about this time that I realised how insistent I could be – I was always very nice and polite, but if I really wanted something, no one could stop me.
My PhD research involved offering an enriched environment to rats, so I made a big animal enclosure that had different floors. By this time I had Isabel, our oldest daughter, so I made all these toys for the animals when she was there with me, and I changed the environment every day and even moved the floors so that it would be a new environment. I had Isabel on my lap when I observed the animals in the large enclosure.
After 14 days of 4 hours of daily exposures to the enriched environment I took living slices of the hippocampus and filled individual hippocampal cells with Lucifer yellow to stain them. That allowed me to visualise and count the spines in 3-D.
I counted spines blind to the group to which each rat belonged, and found that there was a difference between the rats who lived in the enriched environment and those who didn’t. I then trained animals with similar experiences in the water maze, and showed that the animals that had lived in the enriched environment were faster and better at remembering the hidden platform in the water maze. I published two papers on my work on spines, one in PNAS (Proceedings of the National Academy of the Sciences of the United States) and the other in The Journal of Comparative Neurology. I also published a third paper on dorsal and ventral differences in the hippocampus in PNAS.
Children and the Lab
Many people ask me how I managed to do all this work with two small children – Isabel was born in June 1991, right after we started our PhDs (Fig. 2 and Fig. 3), and Ailin was born in 1995 (Fig. 4), right before we completed our PhDs. The answer is that we were so driven to understand the brain that we simply made things work, we could not see any problems – nothing could stop us. From the earliest days, we took the girls to the laboratory – they were both very good children, very well behaved. Or if I couldn’t take them with me, Edvard could take turns watching them. Of course we had nannies and preschool places for the children – both of them attended preschool in Edinburgh, but in the afternoon and in the weekends they often played in the office.

If people objected, I would ask them what was the harm in what I was doing? It wasn’t that I had the freedom – I just assumed I could do things, like take my children to scientific meetings and breast-feed them in public, or bring them to the lab. I just didn’t see the barriers that others might have seen. We were somewhat naïve – we couldn’t imagine that people would object – so people mostly were very nice and didn’t try to stop us.

Edinburgh and London
During our PhD work, Richard Morris had invited Edvard and me to the University of Edinburgh to follow up on our master’s research findings on the difference between the dorsal and the ventral part of the hippocampus, but using chemical lesions instead of aspiration.
We went there several times during our PhDs, and confirmed our earlier results – that the dorsal and the ventral parts of the hippocampus are different. We also conducted another experiment with him on saturating hippocampal synapses with LTP and testing the animal’s ability to learn to find the platform in the water maze. We later completed this work after we came to Trondheim and published the results in Science.
We defended our PhDs in Oslo in December 1995, but by that time we were already in Edinburgh with Richard Morris – we even had the girls in a preschool there. In the spring of 1996, Per Andersen and Morris graciously suggested that we go work with John O’Keefe at University College London to learn how to do single cell recordings. We had already met John O’Keefe in Oslo when we defended our PhDs, because he was the opponent for Ole Paulsen, who was one of Per’s six students (including us) who defended our theses in the same week.
We had an incredible party after the defences, with all of these top international scientists who served as our opponents, with seminars and sleigh ride! Many of the opponents later became important members of our scientific network, which helped us to win status as a centre of excellence from the Research Council of Norway.
The stay in London, in John O’Keefe’s lab, was one of the most learning-rich periods in our lives. John spent an enormous amount of time with us and taught us everything about single cell recordings. He sat with us in the surgery room, showed us how to turn down the tetrodes and do the recordings, how to cluster the data, he talked about the literature – it was all absolutely formative for our future. Edvard had three months with John, and I had just one, in part because of the difficulty of finding care for the children. In Edinburgh we had spots in a preschool for them in Edinburgh only, so in London my brother’s wife, Olaug Andreassen, came for a month to help care for them.
Two Positions and a Lab
At the same time, one position had opened up at the Norwegian University of Science and Technology in Trondheim, and our former supervisor, Terje Sagvolden, encouraged us to apply for it, just for the experience if nothing else. We knew it was a long shot – when we applied we were still months away from defending our PhDs, and we really weren’t thinking that we were ready to settle down just yet.
Suddenly we were called in for an interview, in the autumn of 1995. We told the interview committee that we would not be interested in one position, but Sturla Krekling, the individual at the Department of Psychology who was most involved in the process, really pushed for us and so they offered us two positions. They were in the process of trying to build up the department.
We then said we need a new lab because we wanted to do research – we didn’t just want a salary to teach – so we came to them with a list of all the equipment we needed, the prices, the suppliers, because we had been through the process with Per to build the water maze in Oslo. We also knew what was required to build a combined electrophysiology-water maze lab, we had the experience, and we basically got it all. The only condition was that we begin in August of 1996 so that we could teach.
That completely upended our plans, because we had hoped to stay longer with John O’Keefe, or go to the University of Arizona to work with Carol Barnes and Bruce McNaughton’s memory and hippocampus group. Both Barnes and McNaughton had been involved with us as PhD examiners, and Arizona was a real neuroscience mecca at the time. We really wanted to go there. But that wasn’t possible once we had the offer of positions in Trondheim – the prospect of two jobs and a lab just seemed too good an opportunity to turn down.
(In fairness, we eventually did get a six-week sabbatical in Arizona in 2001, where we learned to do what is called parallel recordings from many dozens of hippocampal cells. It was a technique that was invented by Bruce in the 1990s in Tucson.)
Funding for Collaboration
In parallel with teaching we managed to have our lab operating after a half year of set-up, and after a year or so we had the first results. It took a long time to get data because there was only Edvard and me, and we had to do all the technical aspects of the work along with the actual science – everything from cutting brains to cleaning the rat cages. Our daughters learned to become real “Trøndersk,” an expression that describes the residents of mid-Norway in the counties of Norand Sør-Trøndelag, where Trondheim is located.
One of the first questions we started with was how are the place cells that O’Keefe discovered generated? What is the basis for the place cells in the hippocampus? To answer this question, we applied for – and received – a collaborative grant from the European Commission in 2000 that gave us three years of funding (Fig. 5).

At the same time, we also applied for funding from the Research Council of Norway’s Centre of Excellence programme, a 10-year grant for basic research. This was also approved and as of the December 2002, our group became known as the Centre for the Biology of Memory.
The Centre of Excellence money allowed us to bring internationally recognised researchers to Trondheim for brief but significant periods. We had Menno Witter from the Free University in Amsterdam, Richard Morris from the University of Edinburgh, Bruce McNaughton and Carol Barnes from the University of Arizona, Alessandro Treves from the International School for Advanced Studies in Trieste and Ole Paulsen from Oxford University, all experts in their field, to make a coordinated effort to conduct integrated neural network studies of hippocampal memory. In addition we brought in Randolf Menzel from Freie Universität Berlin who studied neurobiology and spatial memory in the honeybee.
Finding Grid Cells
A few weeks after the centre of excellence grant was announced we published a paper that guided us to search for the spatial signal that resulted in place cells in the hippocampus (Brun, Otnæss, Molden, Steffenach, Witter, Moser and Moser, 2002, in Science). These exciting data suggested that we should search for the spatial signal in structures upstream of the hippocampus – like the entorhinal cortex. That is what helped us to eventually discover grid cells.
In the Brun et al. (2002) paper we asked where the signal to the place cells came from. The reason this question was so intriguing is that the place signal is buried so deeply in the brain, it really can’t be traced back to any sensory input, so how is it made?
We tried to answer this question by making small lesions in the early stages of the hippocampal circuit, in the CA3 area, as a way to interrupt the intrinsic circuit. We then put electrodes in the CA1 area of the hippocampus, which is where most of the place cells had been recorded.
If the place cells were a product of processes that happened at earlier stages of the hippocampus, we should have been able to block those signals by interrupting the hippocampal circuit. But what we found was that we still had spatial signals, even after we made the lesions that should have stopped the signal.
That led us, together with PhD students Marianne Fyhn and Sturla Molden and our colleague Menno Witter, to go to the part of the entorhinal cortex that few other groups had recorded from, the dorso-medial entorhinal cortex. For many reasons people had not worked in the dorsal part of the medial entorhinal cortex before, partly because it was technically difficult, and partly just because of convention.
And this turned out to be the spot, in 2005, where we found grid cells (Fig. 6). Even before we realised that we had discovered a new kind of cell, we realised in 2004 that something was going on in this region with respect to space. The cells there had spatial activity, they had multiple firing peaks and the pattern looked regular – but we really didn’t understand what was going on until we extended the size of the environment that we let the rats roam around in a 200-cm-diameter cylinder. And finally, there it was, a clear hexagonal pattern, and that was the discovery of grid cells.
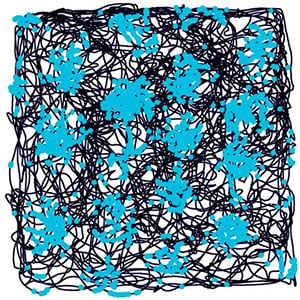
That last sentence makes our discovery sound very simple, but when we first had our results, they were so clear we almost didn’t believe them. We thought perhaps that the hexagonal pattern was an artefact of how we made our measurements. But over time we were convinced that this was in fact how the cells were firing.
We did this research with our students Torkel Hafting, Marianne Fyhn, and Sturla Molden. Marianne came to Trondheim in 2004 to work with us, and Torkel was her boyfriend (and later husband). We offered Torkel a position as a technician in our lab so that they could stay together, and we asked him to work with Marianne on the grid cell project. He later became a postdoc with us. The grid cell results were published in Nature in 2005.
The Brain’s Own Internal Code
Finding grid cells was exciting because it gave us another piece in the puzzle of how we navigate in space. But the larger significance of the find is that for the first time, we were able to see how the brain takes complex information – in this case, information about where we are and how we move in space – to generate its own internal code to make use of that information. There is no grid pattern out in the world – this is just how the brain makes sense of the environment.
The door was opened on this discovery by John O’Keefe’s discovery of place cells, which fire when an animal is at a specific place. In the past, it has been difficult make associations between the firing of neurons deep in the higher parts of the cortex and sensory input, because as the distance between the sensory input and the neuron increases, the firing of the neurons may be triggered by a multitude of converging sensory channels along with intrinsic processes that we don’t really understand.
But O’Keefe and his colleagues found that most hippocampal cells had place fields with distinct firing patterns that collectively allowed the brain to create a map of that environment. Here we had neural activity – the firing of the place cell, deep in the hippocampus – that was clearly associated with a property of the environment, the animal’s location. The discovery of grid cells took this a step further – and it raised new questions that have continued to shape our research, as we seek to understand how grid cells operate and are generated and how they interact with other cell types and in more distant brain structures. Here, we think, lies a key to unlocking the mystery of how the brain computes.
Border Cells and Becoming a Kavli Institute
We have made a great deal of progress since our 2005 discovery. In 2006, for example, we and our colleagues, led by Francesca Sargolini, a postdoc, found cells in the medial entorhinal cortex that tell the animal which direction it is facing, called head direction cells. Previously these had been reported in the dorsal presubiculum by Jim Ranck in 1983.
In 2007, we were selected by the Kavli Foundation as the fourth Kavli neuroscience institute, an award that provides funding for basic research in perpetuity. This meant our lab had two names, the Kavli Institute for Systems Neuroscience and the Centre for the Biology of Memory, and a significant increase in funding and support.
In 2008, we and our colleagues, led by then PhD candidate Trygve Solstad, discovered a third type of entorhinal cell type that we called border cells, because they fire at the edges and boundaries of the local environment. We had already seen these kind of cells when Francesca Sargolini discovered the head and conjunctive grid-head direction cells in the medial entorhinal cortex, but we needed a student to search systematically for them and to do the manipulations that were required to call them border cells – like inserting a wall in the box and seeing that the cell would fire again, along the new wall.
Gamma Oscillations, Map Resolutions and “A Protein”
Parallel with studying border cells, we asked how CA1 cells in the hippocampus were able to cope with receiving what appeared to be conflicting information from the hippocampus itself – from CA3 and from the upstream structure, the entorhinal cortex, at the same time.
Laura Colgin, our then-postdoc, published a paper in 2009 in Nature showing that the brain uses gamma oscillations to route information between grid networks in the entorhinal cortex and the place and memory networks in the hippocampus, which effectively allows the brain to filter out distracting information and focus on one bit of information. We worked for more than five years to finish this amazing story, which shows that passion and persistence go hand-in-hand to produce ground-breaking results.
Also in 2008, with our PhD candidates Kirsten Kjelstrup and Vegard Brun (who were also a couple), we described how the brain makes maps of different resolutions both in the hippocampus and in the entorhinal cortex, thus using place cells and grid cells to create everything from a larger overview map to a finely detailed map. That led the way to the discovery in 2011 led by our colleague Lisa Giocomo that the brain’s ability to make these detailed maps at fine resolution was controlled by a single protein. This last paper was published in Cell with a companion article in the journal Neuron, published by our sister Kavli Institute in New York, headed by our friend and colleague Eric Kandel, whose laboratories had created the mice used in the experiments.
Recording From Many Grid Cells
By 2012, with the ability to record from many grid cells from individual rats, and as many as 186 neurons in one rat called Flekken, we were able to describe how the brain shifts between different map resolutions in a step-wise fashion rather than continuously, and that the brain has at least four difference maps of location, where grid cells are organised into different independent modules in which the scale, orientation and phase relationships are all preserved. These results were published in Nature with another fantastic couple and PhD candidates Hanne and Tor Stensola as first authors.
It had not been possible previously to record from so many cells, which is why we suddenly could show that the hippocampal maps were independent. What was also exciting about these data was that we showed in 2007 that when cells in the hippocampus formed statistically significant different ensembles of active cells – different maps for each environment (Fyhn et al., 2007), this was accompanied with a re-anchoring, or shift of the position of the grid cells relative to the boundaries of the boxes in the different rooms.
The models that had already been developed in 2005, just after we discovered grid cells, suggested that place cell activity was the result of the linear summation of grid cell activity (see Solstad, Moser and Einevoll, 2006, for example). Thus, the discovery of different independent grid modules solved the question we faced in 2007, which was to figure out how grid cells could contribute to the activation of several thousands of different ensembles of hippocampal cells if the grid cells were all part of the same map. Independent grid modules suggested that the entorhinal cortex cell activity could trigger the separation of different memories. However, we have not yet been able to prove that this is the way it works.
Optogenetics and Viruses
To address this issue, we first needed to know that grid cells project to the hippocampus. In 2007, we got an email from Sheng-Jia Zhang that eventually led to us being able to answer this question. He asked if we would be interested in having him come to our lab to set up a molecular lab so that we could use a new molecular tool called optogenetics to address questions we were interested in. He came at a moment when we were very eager to understand the entorhinal – hippocampal circuit so we accepted him, his wife Jing Ye and two students he brought with him, Chenglin Miao and Li Lu. They built a molecular lab with the help of a technician in our lab, Alice Burøy.
In 2013 we were able to use defanged viruses to introduce a gene for a fluorescent marker in addition to a gene for the light-sensitive protein channelrhodopsin. The AAV virus was injected into the hippocampus. It was manipulated in a way so that it would go into the axons of cells projecting to the hippocampus. In this way we could examine which neurons in this part of the brain send axons to place cells in the hippocampus. Thus, when we found grid cells, border cells and other cells in the entorhinal cortex responding to the light at a very short latency (less than 10 ms) coming through an optic fibre that we had inserted into the entorhinal cortex along with the electrodes, we started to believe that these cells sent information to hippocampal place cells. Of course we had to do a set of control experiments to be sure. This is why such ground-breaking data usually takes more than four or five years to write up and get published. This story tells us that the way we like to work is not limited by the lack of methods or tools. Either collaborate with other good labs or set up the method in your own lab to follow your dreams!
Remapping
We have also worked with cells inside the hippocampus. Since we know from the late 1950s (Scoville and Milner, 1957) that the hippocampus is important for encoding and storing episodic memories, it is exciting to understand how the hippocampus solves problems like not mixing similar information and recognizing an object or an environment with only slight changes. These processes are called pattern separation and pattern completion.
In the mid-1980s, Bob Muller and John Kubie showed that small changes in the environment caused large changes in the hippocampal place maps, in a process they called remapping. In 2005, with Stefan Leutgeb, his wife Jill Leutgeb and others, we found that there are at least two types of these kinds of remapping processes. One we called global remapping and the other rate remapping. Rate remapping is recognised by cells keeping their place preference in the test box, but changing the rate when there is a minor change in the environment, such as changed colours on the wall of the test box.
In contrast, global remapping was typically seen if the changes in environments were big, for example that the test rooms were different even though the test boxes were almost identical. In CA3 of the hippocampus, different cells were active or if they were active in both rooms the spatial preference would be shifted, for example a cell with a place field in the middle of the box in the first room could then have a place field in one of the corners of the box in the other room.
This year we published a study, led by PhD candidate Charlotte Alme, showing that the hippocampus can form ten significantly different new maps when rats were tested in ten new rooms over two days. The ten-room experiment demonstrates that when graduate students are passionate about their work and their animals, impossible data collection is made possible.
Teleportation
The remapping experiments in the lab led us to ask what happens if there is a conflict in the animal’s current idea of where it is and the sensory inputs the animals perceives. In order to address this exciting question, Karel Jezek, a postdoc in the lab, ran the animals in a science-fiction inspired experiment. The experiment involved making the animals feel as if they had been teleported from one box to another in a fraction of a second. We were able to create this illusion by training the rats in different boxes that were differentiated only by their lighting schemes. First the rats ran between the boxes, which were connected with a corridor, then the corridor was closed and the rats were tested in only one box where we were able to flip a switch to change the lighting scheme that was associated with one box to the lighting associated with the other box. Thus, while the animal was foraging for chocolate crumbs in one box, we could flip the switch and it would suddenly perceive itself to be located in the other box.
This procedure allowed us to see that each map can be represented in chunks of one theta cycle, or 125 milliseconds, and that the brain’s two maps would compete until one took over, and the hippocampus reliably represented the box that was consistent with the landmarks for that given box. This experiment can typically be compared with a situation we all have experienced: We suddenly wake up in the middle of the night in a hotel room. We are confused, and before we realise that we are not at home but in a hotel, our brains have been switching back a forth between the map for the hotel room and our bedroom at home.
Six Research Groups
Our centre now consists of six research groups, led by Menno Witter (functional anatomy), Yasser Roudi (statistical physics of interference and network organization), Clifford Kentros (transgenic investigation of neural circuits), Jonathan Whitlock (cognitive motor function) and ours (space and memory). Our sixth and newest research group is headed by Emre Yaksi, who studies sensory computations in zebrafish.
The strength of this structure is that we can collaborate across groups. One beautiful example of this was a collaboration between three of the six groups which led us to resolve a question that we had puzzled over for almost eight years. Even with new analyses, new ideas and a lot of discussions of the data both in the lab and at international meetings, we could not figure out what was going on, which is why we did not publish the data.
Menno Witter’s group had discovered that stellate cells did not communicate directly with each other, but with a re-route through inhibitory cells. Thus, the grid cells were surrounded by inhibition. At the same time, Yasser Roudi and some of the people from his group had made a computer model of this network and were able to show that this kind of network could produce a functional grid cell network. The excitation could come from the hippocampus and head direction input. The prediction from this model was thus that by removing the excitation from the hippocampus, the grid cells would change from being grid cells to becoming highly head direction modulated.
This is exactly what we found in our lab – but could not explain before the collaboration. We submitted two manuscripts to Nature Neuroscience and were lucky enough to get them published back-to-back in 2013. We were so happy and proud to finally be able to publish 8-year-old data that had been so intriguing and yet so difficult to decipher – and to be able to work so closely between the different groups at the centre.
The Norwegian Brain Centre
The Norwegian government recognised the importance of neuroscience in Norway by funding the Norwegian Brain Initiative in 2011, which led to us open the Norwegian Brain Centre in 2012 as a collaboration between our lab and research groups working with medical imaging from St Olavs Hospital. The University of Oslo’s Centre for Molecular Biology and Neuroscience is a partner in the Norwegian Brain Initiative. We got a new, big, beautiful lab, with the equipment we need to do ground-breaking science.
We are also working hard to make sure that some of our most important lab workers, the rats and mice, have the best environment a science lab can offer. Most of the animals live in big enriched cages, with toys and nests and together with their cage mates. We also try to let animals that wear electrode implants live with their siblings, since rodents are such social creatures. We have a veterinarian who works full-time for our centre, and four well-educated animal care-takers who love animals. In order for them to always remember that the animals are individuals, they have their own pet animals in the animal quarter. Our goal is that our animals be as happy as possible.
A Second Centre of Excellence
Our lab was also awarded funding at the end of 2012 for a second 10-year-long Centre of Excellence by the Research Council of Norway, just as our first decade-long grant ended. Our new centre is called the Centre for Neural Computation, and is directed by me. It was such a relief when we got that grant. We had just ended the first ten year of CoE funding and we were so worried that we could not keep our lab running at the level we had ambitions for if we did not get new funding.

The day the second CoE funding was announced we were euphoric, and we celebrated (Fig. 7)! And celebrations are important as motivation and glue for the team. The support made us even more inspired and enthusiastic than we had been before, and I was handed an exciting new challenge – to serve as director of a centre that had grown from two people to almost one hundred people from different backgrounds and nations (Fig. 8).

The continued support from our colleagues, the Kavli Institute, the Norwegian University of Science and Technology, the Norwegian government, as well as the city of Trondheim and the county of Sør-Trøndelag has made it possible for us to pursue our dream of unravelling the mysteries of how the brain computes and makes memories and behaviours.
Early on in our careers we realised the importance of bringing in different kinds of expertise in the pursuit of common goals. We also recognise the risks that come from being wedded to only one kind of investigative tool. We’re continually looking to expand our neuroscience “toolbox.” Our six groups are all eager to work together towards our vision: how does the brain generate cognition and mental function. We have an exciting future in front of us.
Two Children and a Lab
Through hard work and persistence together with fantastic colleagues, I have worked towards my dreams and vision from my childhood: to understand how the neural activity in the brain generates behaviour and cognition. By discovering the grid cell network, we suddenly understood something fundamental about the mystery of the brain – how the brain generates a universal map of the environment. The grid cells that do this are located far away from the senses that tell the animal what is out there. In the same deep structure, the entorhinal cortex, we have also discovered other functional cell types that signal the boundaries of the environment, cells that signal the direction the animal is moving in and cells that combine the head direction and grid signals.
We have shown that with changes of sensory input the universal grid map is shifted and anchored differently to the environment – probably changing the active ensembles of hippocampal place cells. Knowing that the hippocampus contains engrams involved in episodic memories, we have shown that this magic circle of entorhinal and hippocampal cell interactions is part of the mechanism for memory. We are working with findings that are the very essence of being a human being: our conscious memories are what make us who we are, and these memories are anchored in space, in knowing where we are in the environment.
Our two daughters have long joked that our lab is like our third child, and in many ways, they are not wrong. We are proud parents to all three of our children. Having real “biological” children in addition to our laboratory “child” has brought an amazing happiness to my life. I think that makes it easy for me to do good science.

I have been lucky to live a fairy tale life, with a partner and a long-time collaborator, Edvard Ingjald Moser, who has supported me and helped me fulfil my dreams ever since we met. We have two wonderful daughters, Isabel Maria Moser and Ailin Marlene Moser. They are wise and loving human beings. Being an internationally recognised scientist brings a lot of adventures and a large network of friends and colleagues across the world (Fig. 9). We have travelled to so many different places and learned so much. Our children have come to think it is quite normal to live like this. Ailin was still a pre-teen when she asked us: why haven’t we visited Easter Island yet? She could also have asked; why haven’t we understood our brains yet?
This autobiography/biography was written at the time of the award and later published in the book series Les Prix Nobel/ Nobel Lectures/The Nobel Prizes. The information is sometimes updated with an addendum submitted by the Laureate.
Nobel Prizes and laureates
See them all presented here.
