Popular information
Popular science background:
Their functional chemistry works wonders (pdf)
Populärvetenskaplig information:
Deras funktionella kemi åstadkommer stordåd (pdf)

The Nobel Prize in Chemistry 2022
Sometimes simple answers are the best. Barry Sharpless and Morten Meldal are awarded the Nobel Prize in Chemistry 2022 because they brought chemistry into the era of functionalism and laid the foundations of click chemistry. They share the prize with Carolyn Bertozzi, who took click chemistry to a new dimension and began using it to map cells. Her bioorthogonal reactions are now contributing to more targeted cancer treatments, among many other applications.
Their functional chemistry works wonders
Ever since the birth of modern chemistry in the eighteenth century, many chemists have used nature as their role model. Life itself is the ultimate proof of nature’s supreme ability to create chemical complexity. The magnificent molecular structures found in plants, microorganisms and animals have spurred researchers to try to construct the same molecules artificially. Imitating natural molecules has often also been an important part in the development of pharmaceuticals, because many of them have been inspired by natural substances.
Centuries of accumulated knowledge in chemistry has proved its worth. Using the sophisticated tools they have developed, chemists can now create the most astounding molecules in their laboratories. However, one challenging problem is that complex molecules must be built in many steps, with each step creating unwanted by-products – sometimes more and sometimes fewer. These by-products must be removed before the process can continue and, for demanding constructions, the loss of material can be so great that hardly anything is left. Chemists often achieve their challenging aims, but the route there can be both time consuming and expensive. The Nobel Prize in Chemistry 2022 is about finding new chemical ideals and letting simplicity and functionality take precedence.
© Johan Jarnestad/The Royal Swedish Academy of Sciences
Chemistry has entered the era of functionalism
Barry Sharpless, who is now being awarded his second Nobel Prize in Chemistry, was the one who started the snowball rolling. Around the turn of the century, he coined the concept of click chemistry for a functional form of chemistry, where molecular building blocks snap together quickly and efficiently. The snowball became an avalanche when Morten Meldal and Barry Sharpless – independently of each other – discovered what has become the crown jewel of click chemistry: the copper catalysed azide-alkyne cycloaddition.
Carolyn Bertozzi developed click reactions that can be used inside living organisms. Her bioorthogonal reactions – which occur without disturbing the normal chemistry of the cell – are used globally to map how cells function. Some researchers are now investigating how these reactions can be used to diagnose and treat cancer, something we will return to. Let us now follow the first of the two threads that lead to the Nobel Prize in Chemistry 2022.
Sharpless believes chemists need new ideals
We start unravelling this thread in 2001, the same year that Barry Sharpless received his first Nobel Prize in Chemistry. However, that was yet to happen when he, in a scientific journal, argued for a new and minimalistic approach in chemistry. He believed it was time for chemists to stop imitating natural molecules. This often resulted in molecular constructions that were very difficult to master, which is an obstacle to the development of new pharmaceuticals.
If a potential pharmaceutical is found in nature, small volumes of the substance can often be manufactured for in vitro testing and clinical trials. However, if industrial production is required at a later stage, a much higher level of production efficiency is necessary. Sharpless used a powerful antibiotic, meropenem, as an example. Six years of chemical development work were necessary to find a way of producing the molecule on a large scale.
Wrangling molecules is expensive
One stumbling block for chemists, according to Barry Sharpless, was the bonds between carbon atoms that are so vital to the chemistry of life. In principle, all biomolecules have a framework of linked carbon atoms. Life has evolved methods for creating these, but it has proven notoriously difficult for chemists. The reason is that carbon atoms from different molecules often lack a chemical drive to form bonds with each other, so they need to be artificially activated. This activation often leads to numerous unwanted side reactions and a costly loss of material.
Instead of trying to wrangle reluctant carbon atoms into reacting with each other, Barry Sharpless encouraged his colleagues to start with smaller molecules that already had a complete carbon frame. These simple molecules could then be linked together using bridges of nitrogen atoms or oxygen atoms, which are easier to control. If chemists choose simple reactions – where there is a strong intrinsic drive for the molecules to bond together – they avoid many of the side reactions, with a minimal loss of material.
Click chemistry – functional green chemistry with huge potential
Barry Sharpless called this robust method for building molecules click chemistry, saying that even if click chemistry cannot provide exact copies of natural molecules, it will be possible to find molecules that fulfil the same functions. Combining simple chemical building blocks makes it possible to create an almost endless variety of molecules, so he was convinced that click chemistry could generate pharmaceuticals that were as fit for purpose as those found in nature, and which could be produced on an industrial scale.
In his publication from 2001, Sharpless listed several criteria that should be fulfilled for a chemical reaction to be called click chemistry. One of these is that the reaction should be able to occur in the presence of oxygen and in water, which is a cheap and environmentally friendly solvent.
He also provided examples of several existing reactions that he believed fulfilled the new ideals he had laid out. However, no one yet knew of the brilliant reaction that has now become almost synonymous with click chemistry – the copper catalysed azide-alkyne cycloaddition. This was about to be discovered in a laboratory in Denmark.
The click reaction that changed chemistry
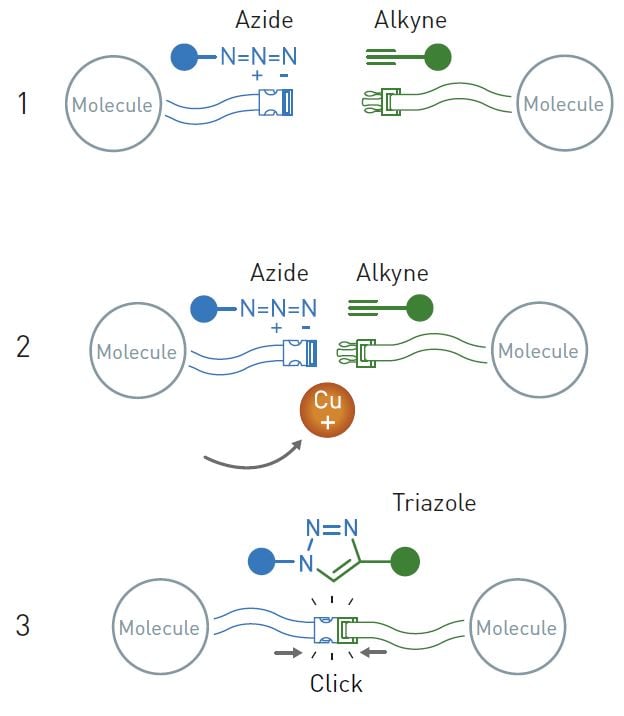
Azides and alkynes react very efficiently when copper ions are added. This reaction is now used globally to link molecules together in a simple manner.
An unexpected substance in Meldal’s reaction vessel
A great deal of decisive scientific progress occurs when researchers least expect it, and this was the case for Morten Meldal. In the early years of this century, he was developing methods for finding potential pharmaceutical substances. He constructed enormous molecular libraries, which could include hundreds of thousands of different substances, and then screened them all to see whether any of them could block pathogenic processes.
While doing this, one day he and his colleagues conducted a purely routine reaction. You don’t need to remember this bit, but their aim was to react an alkyne with an acyl halide. The reaction usually goes smoothly, as long as chemists add some copper ions and perhaps a pinch of palladium as catalysts. But when Meldal analysed what happened in the reaction vessel he found something unexpected. It turned out that the alkyne had reacted with the wrong end of the acyl halide molecule. At the opposite end was a chemical group called an azide (illustrated above). Together with the alkyne, the azide created a ring-shaped structure, a triazole.
This reaction was something special
People who understand some chemistry may know that triazoles are useful chemical structures; they are stable and are found in some pharmaceuticals, dyes and agricultural chemicals, among other things. Because triazoles are desirable chemical building blocks, researchers had previously tried to create them from alkynes and azides, but this led to unwanted by-products. Morten Meldal realised that the copper ions had controlled the reaction so that, in principle, only one substance formed. Even the acyl halide – which really should have bonded to the alkyne – remained more or less untouched in the vessel. It was therefore obvious to Meldal that the reaction between the azide and alkyne was something exceptional.
He first presented his discovery at a symposium in San Diego, in June 2001. The following year, 2002, he published an article in a scientific journal, showing that the reaction can be used to bond together numerous different molecules.
Molecules snap together quickly and efficiently
That same year – independently of Morten Meldal – Barry Sharpless also published a paper about the copper catalysed reaction between azides and alkynes, showing that the reaction works in water and is reliable. He described it as an “ideal” click reaction. The azide is like a loaded spring, where the force is released by the copper ion. The process is robust and Sharpless proposed that chemists can use the reaction to easily link different molecules. He described its potential as enormous. In retrospect, we can see that he was right. If chemists want to link two different molecules they can now, relatively easily, introduce an azide in one molecule and an alkyne in the other. They then snap the molecules together with the help of some copper ions.
Click reactions can be used to create new materials
This simplicity has led to the reaction becoming tremendously popular, both at research laboratories and in industrial development. Among other things, click reactions facilitate the production of new materials that are fit for purpose. If a manufacturer adds a clickable azide to a plastic or fibre, changing the material at a later stage is straightforward; it is possible to click in substances that conduct electricity, capture sunlight, are antibacterial, protect from ultraviolet radiation or have other desirable properties. Softeners can also be clicked into plastics, so they do not leak out later. In pharmaceutical research, click chemistry is used to produce and optimise substances that can potentially become pharmaceuticals.
There are many examples of what click chemistry can achieve. However, something that Barry Sharpless did not predict was that it would be used in living beings. Now we will unravel the second thread in the Nobel Prize in Chemistry 2022.
Bertozzi starts investigating elusive carbohydrates
This thread begins in the 1990s, when biochemistry and molecular biology were undergoing explosive progress. Using new methods in molecular biology, researchers around the world were mapping genes and proteins in their attempts to understand how cells work. There was a pioneering spirit and, every day, new knowledge was generated about areas that had once been terra incognita.
However, one group of molecules received hardly any attention: glycans. These are complex carbohydrates that are built from various types of sugar and often sit on the surface of proteins and cells. They play an important role in many biological processes, such as when viruses infect cells or when the immune system is activated. Glycans are therefore interesting molecules, but the problem was that the new tools for molecular biology could not be used to study them. Anyone who wanted to understand how glycans work therefore faced an enormous challenge. Only a few researchers were prepared to try to climb that mountain – and one of them was Carolyn Bertozzi.
Bertozzi has a bright idea…
In the early 1990s, Carolyn Bertozzi began mapping a glycan that attracts immune cells to lymph nodes. The lack of efficient tools meant that it took four years to get a grip on how the glycan functioned. This challenging process made her dream of something better – and she had an idea. During a seminar, she listened to a German scientist who explained how he had succeeded in getting cells to produce an unnatural variant of sialic acid, one of the sugars that build up glycans. Bertozzi therefore started to wonder whether she could use a similar method to get cells to produce a sialic acid with a type of chemical handle. If the cells could incorporate the modified sialic acid in different glycans, she would be able to use the chemical handle to map them. She could, for example, attach a fluorescent molecule to the handle. The emitted light would then reveal where the glycans were hidden in the cell.
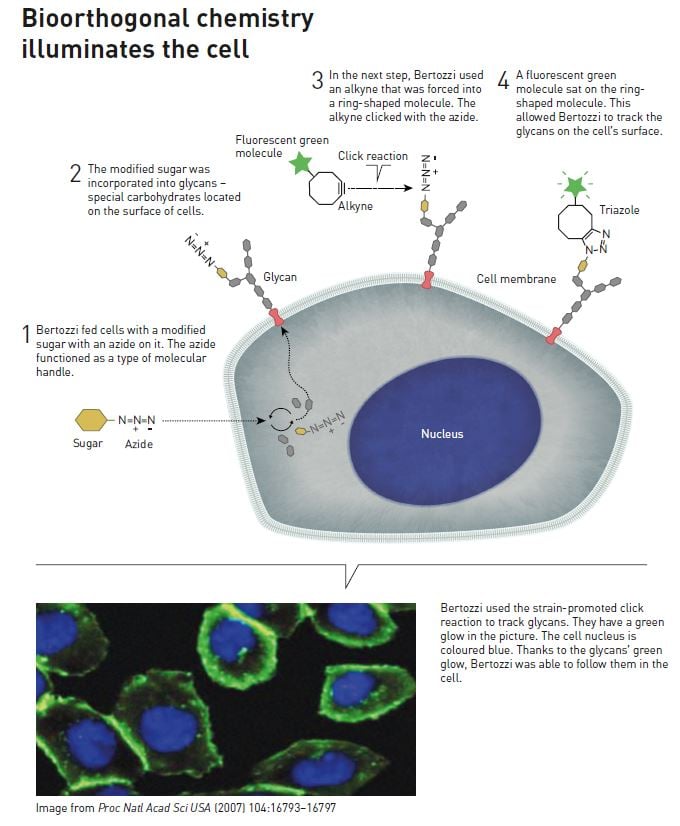
This was the start of long and focused development work. Bertozzi started searching through the scientific literature for chemical handles and a chemical reaction she could use. This was no easy task, because the handle must not react with any other substance in the cell. It had to be insensitive to absolutely everything apart from the molecules she was going to link to the handle. She established a term for this: the reaction between the handle and the fluorescent molecule had to be bioorthogonal.
…and make the hidden glycans reveal themselves
To make a long story short, in 1997 Carolyn Bertozzi succeeded in proving that her idea really worked. The next breakthrough occurred in 2000, when she found the optimal chemical handle: an azide. She modified a known reaction – the Staudinger reaction – in an ingenious manner, and used it to connect a fluorescent molecule to the azide she introduced to the cells’ glycans. Because the azide does not affect the cells, it can even be introduced into living creatures.
With this, she had already given an important gift to biochemistry. With a little chemical creativity, her modified Staudinger reaction can be used to map cells in a variety of ways, but Bertozzi was still not satisfied. She had realised that the chemical handle she used – the azide – had a lot more to give.
Blows new life in an old reaction
At this time, word was spreading among chemists about Morten Meldal’s and Barry Sharpless’ new click chemistry, so Carolyn Bertozzi was well aware that her handle – the azide – can rapidly click onto an alkyne as long as there are available copper ions. The problem is that copper is toxic to living things. So she once again began to dig deep into the literature, and found that back in 1961 it had been shown that azides and alkynes can react in an almost explosive manner – without the help of copper – if the alkyne is forced into a ring-shaped chemical structure. The strain creates so much energy that the reaction runs smoothly.
The reaction worked well when she tested it in cells. In 2004, she published the copper-free click reaction, called the strain-promoted alkyne-azide cycloaddition – and then demonstrated that it can be used to track glycans (see the illustration above).
Click reactions put a spotlight on the cell
This milestone was also the start of something much bigger. Carolyn Bertozzi has continued refining her click reaction, so it works even better in cell environments. In parallel with this, she and many other researchers have also used these reactions to explore how biomolecules interact in cells and to study disease processes.
One area that Bertozzi focuses on is glycans on the surface of tumour cells. Her studies have led to the insight that some glycans appear to protect tumours from the body’s immune system, as they make the immune cells shut down. To block this protective mechanism, Bertozzi and her colleagues have created a new type of biological pharmaceutical. They have joined a glycan-specific antibody to enzymes that break down the glycans on the surface of the tumour cells. This pharmaceutical is now being tested in clinical trials on people with advanced cancer.
Many researchers have also started to develop clickable antibodies that target a range of tumours. Once the antibodies attach to the tumour, a second molecule that clicks to the antibody is injected. For example, this could be a radioisotope that can be used to track tumours using a PET scanner or that can aim a lethal dose of radiation at the cancer cells.
Elegant, clever and novel, but most of all useful
We do not yet know whether these new therapies will work – but one thing is clear: research has just touched on the enormous potential of click chemistry and bioorthogonal chemistry. When Barry Sharpless gave his first Nobel Lecture in Stockholm in 2001, he talked about his childhood, which was coloured by the simple values of the Quakers and has influenced his ideals. He said:
“Elegant” and “clever” were the chemical accolades of choice when I started doing research, just as “novel” is high praise now. Perhaps the Quakers are responsible for me valuing “useful” most.
All four of these words of praise are necessary to do justice to the chemistry for which he, Carolyn Bertozzi and Morten Meldal have laid the foundation. In addition to being elegant, clever, novel and useful, it also brings the greatest benefit to humankind.
Further reading
Additional information on this year’s prizes, including a scientific background in English, is available on the website of the Royal Swedish Academy of Sciences, www.kva.se, and at www.nobelprize.org, where you can watch video footage of the press conferences, the Nobel Lectures and more. Information on exhibitions and activities related to the Nobel Prizes and the Prize in Economic Sciences is available at www.nobelprizemuseum.se
The Royal Swedish Academy of Sciences has decided to award the Nobel Prize in Chemistry 2022 to
CAROLYN R. BERTOZZI
Born 1966 in Boston, MA, USA.
PhD 1993 from UC Berkeley, CA, USA. Anne T. and
Robert M. Bass Professor at
Stanford University, CA, USA
and Investigator, Howard Hughes Medical Institute, USA.
MORTEN MELDAL
Born 1954 in Copenhagen, Denmark.
PhD 1986 from Technical University of
Denmark, Lyngby, Denmark.
Professor at University of
Copenhagen, Denmark.
K. BARRY SHARPLESS
Born 1941 in Philadelphia, PA, USA.
PhD 1968 from Stanford University,
CA, USA. W. M. Keck Professor at
Scripps Research, La Jolla, CA,
USA.
“for the development of click chemistry and bioorthogonal chemistry”
Science Editors: Peter Brzezinski, Olof Ramström, Johan Åqvist, the Nobel Committee for Chemistry
Text: Ann Fernholm
Translator: Clare Barnes
Illustrations: © Johan Jarnestad/The Royal Swedish Academy of Sciences
Editor: Marianne Nordenlöw
© The Royal Swedish Academy of Sciences
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 12 laureates' work and discoveries range from proteins' structures and machine learning to fighting for a world free of nuclear weapons.
See them all presented here.
