Popular information
Popular science background:
They added colour to nanotechnology (pdf)
Populärvetenskaplig information:
De satte färg på nanotekniken (pdf)

The Nobel Prize in Chemistry 2023
Moungi G. Bawendi, Louis E. Brus and Aleksey Yekimov are awarded the Nobel Prize in Chemistry 2023 for the discovery and development of quantum dots. These tiny particles have unique properties and now spread their light from television screens and LED lamps. They catalyse chemical reactions and their clear light can illuminate tumour tissue for a surgeon.
They added colour to nanotechnology
“Toto, I’ve a feeling we’re not in Kansas anymore,” is a classic quote from the film The Wizard of Oz. Twelve-year-old Dorothy faints onto her bed when her house is swept away by a powerful tornado, but when the house lands again and she steps outside the door, her dog Toto in her arms, everything has changed. Suddenly she is in a magical, technicolour world.
If an enchanted tornado were to sweep into our lives and shrink everything to nano dimensions, we would almost certainly be as astonished as Dorothy in the land of Oz. Our surroundings would be dazzlingly colourful and everything would change. Our gold earrings would suddenly glimmer in blue, while the gold ring on our finger would shine a ruby red. If we tried to fry something on the gas hob, the frying pan might melt. And our white walls – whose paint contains titanium dioxide – would start generating lots of reactive oxygen species.
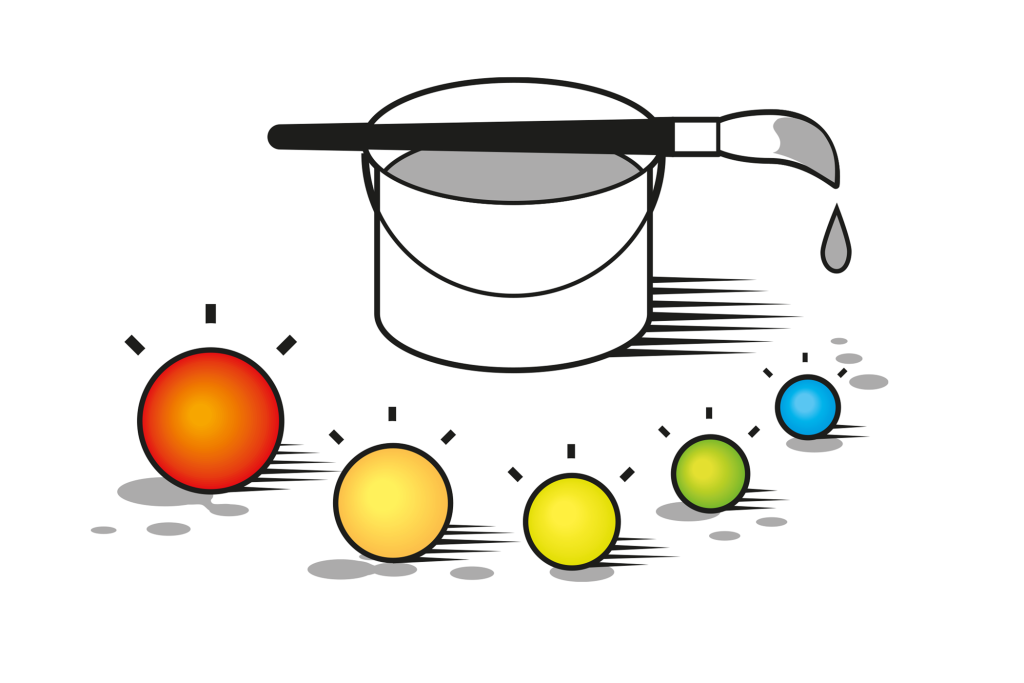
Size matters on the nanoscale
In the nanoworld, things really do behave differently. Once the size of matter starts to be measured in millionths of a millimetre, strange phenomena start to occur – quantum effects – that challenge our intuition. The 2023 Nobel Laureates in Chemistry have all been pioneers in the exploration of the nanoworld. In the early 1980s, Louis Brus and Aleksey Yekimov succeeded in creating – independently of each other – quantum dots, which are nanoparticles so tiny that quantum effects determine their characteristics. In 1993, Moungi Bawendi revolutionised the methods for manufacturing quantum dots, making their quality extremely high – a vital prerequisite for their use in today’s nanotechnology.
Thanks to the work of the laureates, humanity is now able to utilise some of the peculiar properties of the nanoworld. Quantum dots are now found in commercial products and used across many scientific disciplines, from physics and chemistry to medicine – but we are getting ahead of ourselves. Let’s uncover the background to the Nobel Prize in Chemistry 2023.
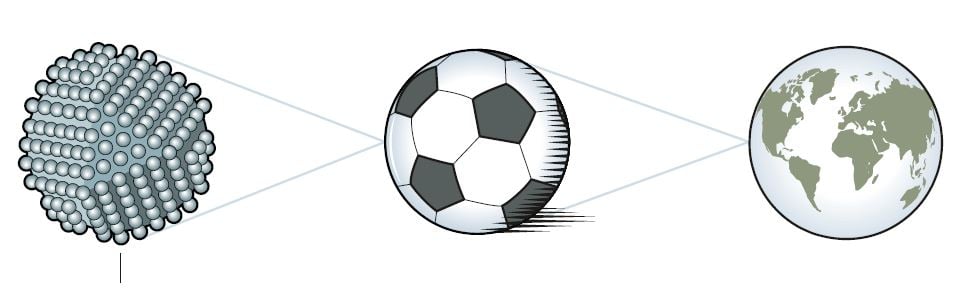
For decades, quantum phenomena in the nanoworld were just a prediction
When Aleksey Yekimov and Louis Brus produced the first quantum dots, scientists already knew that they could – in theory – have unusual characteristics. In 1937, the physicist Herbert Fröhlich had already predicted that nanoparticles would not behave like other particles. He explored the theoretical consequences of the famous Schrödinger equation, which shows that when particles become extremely small there is less space for the material’s electrons. In turn, the electrons – which are both waves and particles – are squeezed together. Fröhlich realised that this would result in drastic changes to the material’s properties.
Researchers were fascinated by this insight and, using mathematical tools, they succeeded in predicting numerous size-dependent quantum effects. They also worked to try to demonstrate them in reality, but this was easier said than done because they needed to sculpt a structure that was about a million times smaller than a pinhead.
Few people thought quantum effects could be utilised
Still, in the 1970s, researchers did succeed in making such a nanostructure. Using a type of molecular beam, they created a nano-thin layer of coating material on top of a bulk material. Once the assembly was complete, they were able to show that the coating’s optical properties varied depending on how thin it was, an observation that matched the predictions of quantum mechanics.
This was a major breakthrough, but the experiment required very advanced technology. Researchers needed both an ultra-high vacuum and temperatures close to absolute zero, so few people expected that quantum mechanical phenomena would be put to practical use. However, now and again science offers up the unexpected and, this time, the turning point was due to studies of an ancient invention: coloured glass.
A single substance can give glass different colours
The oldest archaeological finds of coloured glass are from several thousand years ago. Glassmakers have tested their way to an understanding of how glass can be produced in all the colours of the rainbow. They added substances such as silver, gold and cadmium and then played with different temperatures to produce beautiful shades of glass.
In the nineteenth and twentieth centuries, when physicists started to investigate the optical properties of light, the glassmakers’ knowledge was put to use. Physicists could use coloured glass to filter out selected wavelengths of light. To optimise their experiments, they started to produce glass themselves, which led to important insights. One thing they learned was that a single substance could result in completely differently coloured glass. For example, a mixture of cadmium selenide and cadmium sulphide could make glass turn either yellow or red – which one it became depended on how much the molten glass was heated and how it was cooled. Eventually, they were also able to show that the colours came from particles forming inside the glass and that the colour depended on the particles’ size.
This was more or less the state of the knowledge at the end of the 1970s, when one of this year’s laureates, Aleksey Yekimov, a recent doctoral graduate, started working at the S. I. Vavilov State Optical Institute in what was then the Soviet Union.
Aleksey Yekimov maps the mysteries of coloured glass
The fact that a single substance could result in different coloured glass interested Aleksey Yekimov, because it is actually illogical. If you paint a picture in cadmium red, it will always be cadmium red, unless you mix in other pigments. So how could a single substance give glass of different colours?
During his doctoral degree, Yekimov studied semiconductors – important components in microelectronics. In this field, optical methods are used as diagnostic tools for assessing the quality of semiconducting material. Researchers shine light on the material and measure the absorbance. This reveals what substances the material is made from and how well-ordered the crystal structure is.
Yekimov was familiar with these methods, so he began using them to examine coloured glass. After some initial experiments, he decided to systematically produce glass that was tinted with copper chloride. He heated the molten glass to a range of temperatures between 500°C and 700°C, varying the heating time from 1 hour to 96 hours. Once the glass had cooled and hardened, he X-rayed it. The scattered rays showed that tiny crystals of copper chloride had formed inside the glass and the manufacturing process affected the size of these particles. In some of the glass samples they were only about two nanometres, in others they were up to 30 nanometres.
Interestingly, it turned out that the glass’ light absorption was affected by the size of the particles. The biggest particles absorbed the light in the same way that copper chloride normally does, but the smaller the particles, the bluer the light that they absorbed. As a physicist, Yekimov was well acquainted with the laws of quantum mechanics and quickly realised that he had observed a size-dependent quantum effect (figure 3).
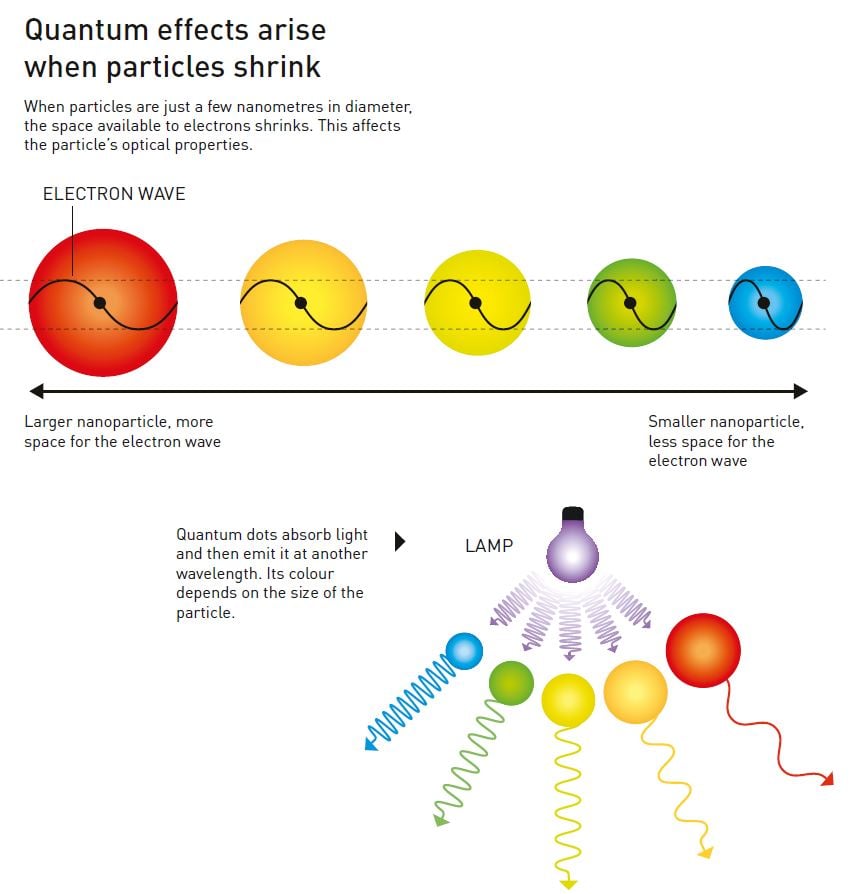
This was the first time someone had succeeded in deliberately producing quantum dots – nanoparticles that cause size-dependent quantum effects. In 1981, Yekimov published his discovery in a Soviet scientific journal, but this was difficult for researchers on the other side of the Iron Curtain to access. Therefore, this year’s next Nobel Prize Laureate in Chemistry – Louis Brus – was unaware of Aleksey Yekimov’s discovery when, in 1983, he was the first researcher in the world to discover size-dependent quantum effects in particles floating freely in a solution.
Brus shows that the strange properties of particles are quantum effects
Louis Brus was working at Bell Laboratories in the US, with the long-term aim of making chemical reactions happen using solar energy. To achieve this, he was using particles of cadmium sulphide, which can capture light and then utilise its energy to drive reactions. The particles were in a solution and Brus made them very small, because this gave him a larger area on which the chemical reactions could take place; the more a material is chopped up, the greater the surface area it will expose to its surroundings.
During his work with these tiny particles, Brus noticed something strange – their optical properties changed after he had left them on the lab bench for a while. He guessed that this could be because the particles had grown, so to confirm his suspicions he produced cadmium sulphide particles that were just about 4.5 nanometres in diameter. Brus then compared the optical properties of these newly made particles with those of the larger particles, which had a diameter of about 12.5 nanometres. The larger particles absorbed light at the same wavelengths as cadmium sulphide generally does, but the smaller particles had an absorption that shifted towards blue (figure 3).
Just like Yekimov, Brus understood that he had observed a size-dependent quantum effect. He published his discovery in 1983 and then started investigating particles made from a range of other substances. The pattern was the same – the smaller the particles, the bluer the light they absorbed.
The periodic table gained a third dimension
This is where you may be tempted to ask “Why does it matter if a substance’s absorbance is slightly more towards blue? Why is that so amazing?”
Well, the optical changes revealed that the substance’s characteristics had completely changed. A substance’s optical properties are governed by its electrons. The same electrons also govern the substance’s other properties, such as its ability to catalyse chemical reactions or conduct electricity. So when researchers detected the changed absorption they understood that, in principle, they were looking at an entirely new material.
If you want to understand the magnitude of this discovery, you can imagine that the periodic table suddenly gained a third dimension. An element’s properties are not only affected by the number of electron shells and how many electrons there are in the outer shell but, at the nano level, size also matters. A chemist who wanted to develop a new material thus had another factor to play with – of course this tickled researchers’ imaginations!
There was just one problem. The methods Brus had used to fabricate nanoparticles generally resulted in unpredictable quality. Quantum dots are tiny crystals (figure 2) and the ones that could be produced at that time often contained defects. They were also of varying sizes. It was possible to control how the crystals were formed so the particles had a given average size, but if researchers wanted all the particles in a solution to be about the same size they had to sort them after they were made. This was a difficult process that hindered development.
Moungi Bawendi revolutionises the production of quantum dots
This was a problem that this year’s third Nobel Prize Laureate in Chemistry decided to solve. Moungi Bawendi started his postdoctoral training at Louis Brus’ laboratory in 1988, where intensive work was underway to improve the methods used to produce quantum dots. Using a range of solvents, temperatures and techniques, they experimented with a variety of substances to try and form well-organised nanocrystals. And the crystals were getting better, but were still not good enough.
However, Bawendi did not give up. When he started working as a research leader at the Massachusetts Institute of Technology, MIT, he continued his efforts to produce higher quality nanoparticles. The major breakthrough came in 1993, when the research group injected the substances that would form nanocrystals into a heated and carefully chosen solvent. They injected as much of the substances as was necessary to precisely saturate the solution, which led to tiny crystal embryos beginning to form simultaneously (figure 4).
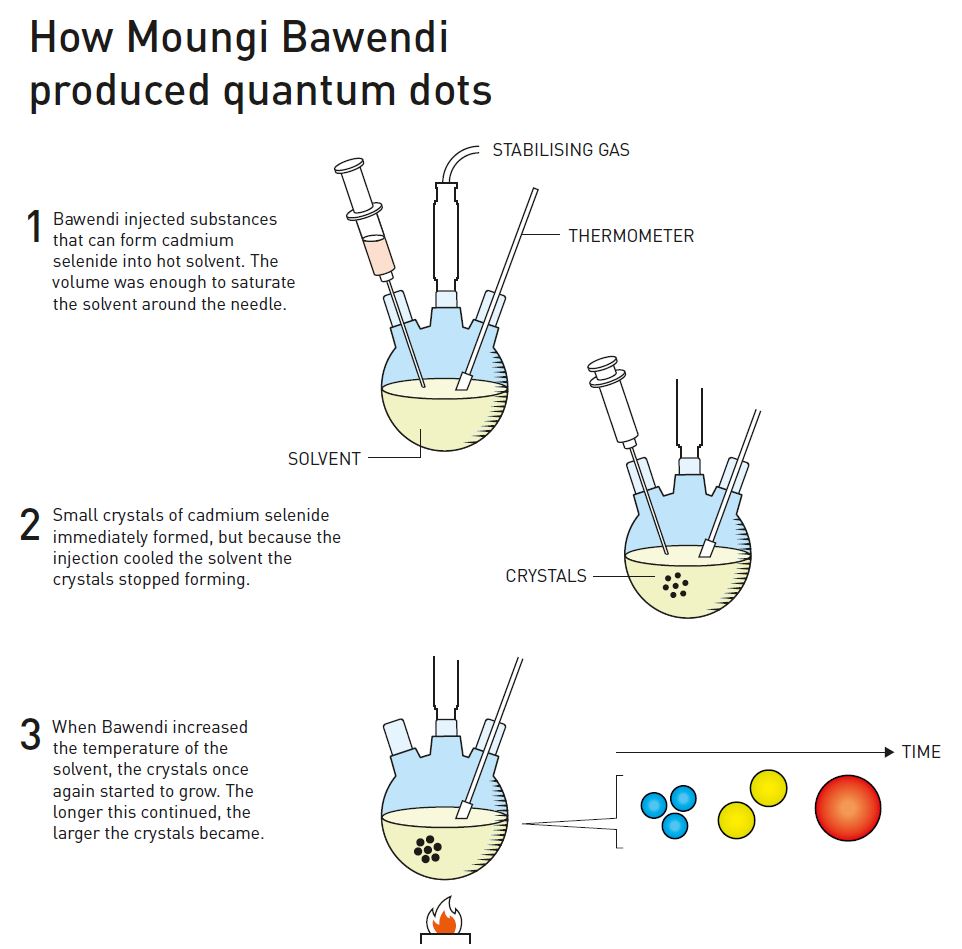
Then, by dynamically varying the temperature of the solution, Moungi Bawendi and his research group succeeded in growing nanocrystals of a specific size. During this phase, the solvent helped give the crystals a smooth and even surface.
The nanocrystals that Bawendi produced were almost perfect, giving rise to distinct quantum effects. Because the production method was easy to use, it was revolutionary – more and more chemists started working with nanotechnology and began to investigate the unique properties of quantum dots.
The luminous properties of quantum dots find commercial uses
Thirty years later, quantum dots are now an important part of nanotechnology’s toolbox and are found in commercial products. Researchers have primarily utilised quantum dots to create coloured light. If quantum dots are illuminated with blue light, they absorb the light and emit a different colour. Modifying the size of the particles makes it possible to determine exactly what colour they should glow (figure 3).
The luminous properties of quantum dots are utilised in computer and television screens based on QLED technology, where the Q stands for quantum dot. In these screens, blue light is generated using the energy-efficient diodes that were recognised with the Nobel Prize in Physics 2014. Quantum dots are used to change the colour of some of the blue light, transforming it into red or green. This makes it possible to produce the three primary colours of light needed in a television screen.
Similarly, quantum dots are used in some LED lamps to adjust the cold light of the diodes. The light can then become as energising as daylight or as calming as the warm glow from a dimmed bulb. The light from quantum dots can also be used in biochemistry and medicine. Biochemists attach quantum dots to biomolecules to map cells and organs. Doctors have begun investigating the potential use of quantum dots to track tumour tissue in the body. Chemists instead use the catalytic properties of quantum dots to drive chemical reactions.
Quantum dots are thus bringing the greatest benefit to humankind, and we have just begun to explore their potential. Researchers believe that in the future quantum dots can contribute to flexible electronics, miniscule sensors, slimmer solar cells and perhaps encrypted quantum communication. One thing is certain – there is a lot left to learn about amazing quantum phenomena. So if there’s a 12-year-old Dorothy out there looking for adventure, the nanoworld has a great deal to offer.
Further reading
Additional information on this year’s prizes, including a scientific background in English, is available on the website of the Royal Swedish Academy of Sciences, www.kva.se, and at www.nobelprize.org, where you can watch video from the press conferences, the Nobel Lectures and more. Information on exhibitions and activities related to the Nobel Prizes and the Prize in Economic Sciences is available at www.nobelprizemuseum.se.
The Royal Swedish Academy of Sciences has decided to award the Nobel Prize in Chemistry 2023 to
MOUNGI G. BAWENDI
Born 1961 in Paris, France. PhD 1988 from University of Chicago, IL, USA. Professor at Massachusetts Institute of Technology (MIT), Cambridge, MA, USA.
LOUIS E. BRUS
Born 1943 in Cleveland, OH, USA. PhD 1969 from Columbia University, New York, NY, USA. Professor at Columbia University, New York, NY, USA.
ALEKSEY YEKIMOV
Born 1945 in the former USSR. PhD 1974 from Ioffe Physical-Technical Institute, Saint Petersburg, Russia.
Formerly Chief Scientist at Nanocrystals Technology Inc., New York, NY, USA.
“for the discovery and synthesis of quantum dots”
Science Editors: Peter Brzezinski, Heiner Linke, Johan Åqvist, the Nobel Committee for Chemistry
Text: Ann Fernholm
Translator: Clare Barnes
Illustrations: Johan Jarnestad
Editor: Alicia Hegner
© The Royal Swedish Academy of Sciences
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 12 laureates' work and discoveries range from proteins' structures and machine learning to fighting for a world free of nuclear weapons.
See them all presented here.
