George A. Olah – Photo gallery
1994 laureates on stage at the Nobel Prize award ceremony at the Stockholm Concert Hall on 10 December 1994. From left: physic laureates Bertram N. Brockhouse and Clifford G. Shull, chemistry laureate George A. Olah, medicine laureates Alfred G. Gilman and Martin Rodbell, literature laureate Kenzaburo Oe and economic sciences laureates John C. Harsanyi, John F. Nash Jr. and Reinhard Selten.
Nobel Foundation. Photo: Lars Åström
George A. Olah and HM Queen Silvia of Sweden at the Nobel Prize banquet in the Stockholm City Hall, 10 December 1994.
Nobel Foundation. Photo: Lars Åström
George A. Olah delivering his speech of thanks at the Nobel Prize banquet in the Stockholm City Hall, 10 December 1994.
Nobel Foundation. Photo: Lars Åström
1994 laureates assembled at the Swedish Academy in Stockholm in December 1994. Back row: Medicine laureates Martin Rodbell and Alfred G. Gilman, economic sciences laureate John F. Nash Jr., chemistry laureate George A. Olah, economic sciences laureate Reinhard Selten and physic laureate Clifford G. Shull. Front row: Physic laureate Bertram N. Brockhouse, literature laureate Kenzaburo Oe and economic sciences laureate John C. Harsanyi.
Photo from the Lars Åström archive
The Nobel Prize in Chemistry 1994
George A. Olah – Other resources
Links to other sites
George A. Olah – Nobel Lecture
Nobel Lecture, December 8, 1994
My Search for Carbocations and Their Role in Chemistry
Read the Nobel Lecture
Pdf 2.25 MB
George A. Olah – Banquet speech
George A. Olah’s speech at the Nobel Banquet, December 10, 1994
Your Majesties, Your Royal Highnesses, Ladies and Gentlemen,
I am most grateful for the honor bestowed on me today. Although receiving the Nobel Prize is the greatest satisfaction any scientist can experience, I consider it not only a personal acknowledgment, but also that of all my students, associates and colleagues whose dedicated work over the years allowed my field of chemistry, which is not frequently highlighted in public, to be recognized.
There are many facets of chemistry. Mankind’s drive to uncover the secrets of life’s processes and use this knowledge led to spectacular advances in the biological and health sciences. Chemistry richly contributes to this by helping our understanding at the molecular level. Chemistry is, however, and always will be a central science of its own.
Chemists make compounds and strive to understand their reactions. My own interest lies in the chemistry of the compounds of the elements carbon and hydrogen, called hydrocarbons. These make up petroleum oil and natural gas and thus are in many ways essential for every day life. They generate energy and heat our houses, fuel our cars and airplanes and are raw materials for most manmade materials ranging from plastics to pharmaceuticals. Many of the chemical reactions essential to hydrocarbons are catalyzed by acids and proceed through positive ion intermediates, called carbocations.
To be able to prepare and study these elusive species in stable form acid billions of times stronger than concentrated sulfuric acid were needed (so called superacids). Some substituted carbocations, however, are remarkably stable and are even present in nature. You may be surprised to learn that the fine red wine we drank tonight contained carbocations which are responsible for the red color of this natural 12% or so alcoholic solution. I hope you enjoyed it as much as I did.
Chemistry does not always enjoy the best of reputation. Many of our plants and refineries are still potentially dangerous and may pollute their surroundings. At the same time our society enjoys a high standard of living not in small measure through the results of chemistry, which few would give up. I believe that chemistry can and will be able to bring about an equilibrium between mankind’s needs and our environmental concerns. Chemistry will continue to benefit mankind in the spirit of Alfred Nobel, a fellow chemist whose example continues to inspire us all.
George A. Olah – Interview
Interview transcript
I’m sitting here in Lindau in Southern Bavaria, this is the 50th anniversary of the Lindau so called Nobelpreisträgertagungen and I am sitting here with George Olah who received the Nobel Prize in Chemistry in 1994. I would like to ask you first, Professor Olah, could you tell us a little bit about how and why you became a scientist.
George A. Olah: I was born and grew up and lived in Hungary until I was 29. When I was growing up and going to school, I must confess I had absolutely no interest in science. As a matter of fact it never crossed my mind that science would be an area I would be involved in. I had much interest in history, literature, languages, even philosophy. At the end of World War II, in war-devastated Hungary in central Europe, when the time came to enter university, it certainly became clear to me that I’d better try to get into a profession that I also could make a living. When took my first chemistry class I fell in love with chemistry – don’t ask me why because I can’t explain why you are falling in love, but I am still in love with it. It’s maybe disappointing, but I was not one of these wunderkind who at age ten already knew exactly what he wanted to do and studied /- – -/ before this.
Maybe I should mention Eugene Wigner who was also a Hungarian born physicist. I read once he wrote about his life and when he was finishing high school his father was a businessman sat down with him and asked what he wanted to become. He said that he would like to become a theoretical physicist and his father, who I guess never had heard of theoretical physics, answered ‘And tell me how many jobs in this little country of Hungary are for theoretical physicists?’ His son, who was an honest guy, said ‘I guess two, maybe three.’ At which time the conversation was terminated his father enrolled him as a chemical engineer, but later on he shifted gears.
Can you say a few words about your family environment, what kind of family did you grew up in?
I was born in a György middle class family, my father was a lawyer and my mother was a homemaker. To my best knowledge nobody in my family ever had any interest in science. I was fortunate enough that I got a fairly schooling, education – much was said about the schools of Budapest and the number of people came out, not only in the sciences but in other areas – musicians, conductors and so on. There was a wonderful music school in Budapest, founded by Franz Liszt, but there was a number of these gymnasien – combined middle and high schools – so I have gone to one of them, not to the one where all my well-known physicist compatriots have gone, but this was run by a Catholic order called the Piarist Brothers. It was really a gear to a general education, heavily in the humanities and so on.
I never heard the name ever mentioned, and I am quite sure about it during my eight years in this school, of George de Hevesy who was a student of the same school who won the Nobel Prize in Chemistry I guess in 1944 or 1945 – maybe there was some tradition, but if it was it was hidden. I guess it served me very well, that it was a very well-balanced general education. I am sorry to say I can’t remember my chemistry teacher’s name, but I remember my physics teacher whose name was Joseph /- – -/, who was a very inspiring teacher. Later I understand he became well known because he became a university professor and introduced on television popular science, in Hungary, but he certainly had a substantial influence as young boys. Also, I never considered to go into physics.
Could you say a little bit about how you came to do the work that you eventually were awarded the Nobel Prize for? Did you have any inspiration, a special idea, was there some new development of apparatus or how did it come about?
I am a chemist who is mostly interested in compounds of the element carbon which is a fairly central element on Earth. Table salt, sodium chloride, is composed of a sodium ketone positive ion and a chloride ion, but carbon compounds are supposed to be different and their ability to form ionic compounds was doubted for a long while. I was studying reactions which involved chemistry, which could have involved ionic carbon compounds – nobody really knew – it was suspected. For long years I had an interest to pursue this chemistry but also with an eye to try to find out how this really goes. It’s not a question of intuition overnight, that you wake up and you have a tremendous idea – some people may have it – it wasn’t with me, but through a fairly long struggle eventually I was lucky to find systems in which these long elusive positive ions of carbons which they call carbocations – carbon is the element and cations is the positive ions – were observable and as the Nobel Committee stated I give supposedly long life to these ions.
There were many things I don’t want to bore with chemistry, but in order to do this it was necessary to use very acidic systems which now are called superacids. When I say very acidic, say your car battery has sulphuric acid in it, and when I grew up, maybe even now in most schools, school children are taught that sulphuric acid is a strong acid. The acid in which my chemistry was possible has acidities which are say a trillion times stronger than sulphuric acid. These are very big numbers, very little meaning, I don’t know whether Sweden has any national debt to hide this, but the US has a national debt I guess of about 5 trillion dollars, so my acids are in this range.
Receiving the Nobel Prize – did that mean for you any big change in your daily life, in your direction of study or was it just another one of those prizes that you have received earlier?
Obviously, anybody who receives this prize tells you that it has an effect. Look, one thing is I wouldn’t be sitting here interviewed by you if I wouldn’t have received the Nobel Prize. On the other hand, I was quite well established in my doubt that it basically … I am still a working scientist and I still love to do this, and I’m also blessed with a wonderful wife who keeps me very down to Earth. I haven’t changed, I hope, as an individual. I work harder than ever because with the Nobel Prize are coming new responsibilities which I try to perform, but I still, my primary life hasn’t changed.
Professor Olah, would you say that your work that you received the Nobel Prize for has a special meaning, that it has a special importance for applications, for chemistry or for society?
Of course all scientists believe what they are doing is significant, but I mentioned that my work still center the chemistry of carbon compound. There was a well-known famous German chemist in the middle of the last century called Kekulé, and one of Kekulé’s major contributions to chemistry was his concept, which still is guiding, chemistry which is generally called organic chemistry, the chemistry of carbon and /- – -/ compound. The carbon can attach itself simultaneously to not more than a maximum of four other atoms or groups. In studying these positively charged species of carbon we realised through a series of investigation that with these systems carbon can attach five atoms or groups, sometimes six, and recently we showed even seven.
This doesn’t violate the fundamental rule of what in chemistry is called the octet rule, so you can’t have more than eight electrons surrounding carbon at any time, but on the other hand, if I mention the simplest carbon hydrating compound, hydrocarbon, is methane, CH4. With our very strong acids we can attach a proton to methane and CH5+ is not a fictional species anymore, it’s a very realistic and quite intriguing species which has substantial bearing on fundamental chemistry in general, but in a practical way. This chemistry opened up possibilities to activate and react hydrocarbons, natural gas, and all are really just mixtures of hydrocarbons. The chemistry we developed and are still developing, to take say methane, a natural gas, and transform it into all kinds of useful products through this new type of chemistry.
What did you think about when you first received the news of your Nobel Prize?
You know it’s a time difference – this was early in the morning – we are early risers so we were up and having breakfast and you get this proverbial phone call. Obviously, it takes a while to sink in. You are obviously gratified and elated – you really don’t know what this all means – and then all hell broke loose. Then we had this wonderful week in Stockholm when we were floating on adrenaline – it takes a while that it settles in. I already told you that it is a wonderful gratifying thing, on the other hand you should keep your proportion. The fact that you get a prize really isn’t making you overnight some type of a different person.
Do you think you could say three words to describe yourself?
Three, it may be five. I am a human being interested in science. In the sequence. The second one is that I learned one thing very useful, it is that I gladly admit how little I know. The third one is that whereas that you don’t plan that your work has practical uses it’s a great pleasure when you can apply some of your knowledge to do something which may be useful for the future.
What do you do when you relax?
My wife sits here so I must be very careful answering you. I am blessed with a wonderful family and we have two wonderful grandchildren, but I must tell you that I am still a very hard worker. My life is around science, although one thing the prize did to me was that my wife and some friends convinced me to write something which was originally supposed to be about my life experience, but it ended up to a great degree about reflections. I told you I was much interested, growing up, in many things, so I spent five years really reading very hard, filling in lots of gaps – not the chemistry but science, philosophy, history and so on – and I greatly enjoyed doing this.
Have you written it? Is it published?
It should come out this year. As it happened the great Greek thinker /- – -/ they were able to cover everything, the physical world and the spiritual world. Then things became very complicated. By the end of the 19th century many philosophers really gave up because I guess they tried to avoid to be embarrassed about the limited knowledge of the physical world. Then, it looks like that physicists – please forgive me mentioning this – particularly practical physicists took over and they believe they have all the answers. I personally believe we probably never will have all the answers, but maybe a few chemists, Eigen and Prigogine and others express some views, not too many, so I try to put in my /- – -/, maybe a big failure but I enjoyed doing it.
Do you teach at all, and if you teach – do you think that teaching gives you something back?
I still teach, and I love teaching. It may surprise you, but I am teaching presently in this fall again, undergraduates, on a topic which is concerned with the relationship between science, arts and even economics. Whereas it is a very informal course, the text we are using is Goethe’s Faust as something which was written by a great poet. The story, at least the first part, is a chemist story, or an alchemist story, and the second part is that whereas the alchemist couldn’t make gold, but paper money was about invented at the time and he puts paper money instead of gold saying that from nothing some value is created. I enjoyed it very much and I learned more than my students.
Thank you very much. The goal of conversation for your life as a scientist – do you think conversation with others is an important factor for your scientific work?
It is absolutely important. I was never fortunate enough to have been in a milieu /- – -/ than in an institution which was one of the leading outstanding institutions in the world. Scientifically, I am a grandson of Emil Fischer who was one of the great organic chemists of our time, and my professor brought back to little Hungary some of Fischer’s concepts. That is for any scientist essential to have contact, free exchange and as we said ‘kicking around ideas’. You do this all the time with your students. I consider the greatest blessing to be a university professor. It keeps you young, your students are your wider scientific family and in this given /- – -/ I hope that I am inspiring them a little. At the same time they keep me active and going.
Can you give an example of a creative milieu – an example, you don’t have to define it. Can you think of some place?
Obviously there are wonderful creative milieus because there are these wonderful schools and centers of culture and so on. As I told you I never was lucky enough, so I needed to create my own milieu. Maybe I am strong, /- – -/ and lucky but I was able to do it and I have my students today, in a small way, I can too provide. I think my role as a professor is a catalyst and somebody who tries to provide them a milieu where they can pursue their work and study relatively shielded from what they will later be exposed into.
It is said that mathematics is beautiful. Is there a beauty also in chemistry?
The beauty is in the eye of the beholder. For me, certainly there is. Mathematics I guess is a universal language of all the sciences. For mathematics I think you must have some born talent. I am not so sure for chemistry you need to have a born talent. Probably it’s helpful that you need to have an enquiring mind. Creativity I think, what we discussed, is to me very difficult to define, because if somebody can define creativity, he or she is probably isn’t very creative. I think an artist or a great painter, sculptor can define for you or you never think the word creativity. But to me chemistry certainly has a great degree of beauty. There are people who find symmetry and write books about it, but to me the beauty is that if you find out some underlined principles then you can build on it. It’s a wonderful experience.
Why are so many Nobel Laureates of Hungarian origin?
I can’t answer you. I don’t think there are special Hungarian genes for science or music or whatever. Probably, and this is only … I never knew the rest of Hungary, only Budapest. Probably in part, it was due to the fact that there were some good schools, there was a basis for it. Many of the Hungarian scientists /- – -/ got recognition did their work outside of Hungary. Albert Szent-Györgyi worked there and I did at least my initial work in Hungary. Being in a small country maybe there is this extra initiative that you try to prove that even in a small poor country you can do something. But I really don’t think that Hungarians are very different from anybody else, we are all human beings.
Did you find any typos in this text? We would appreciate your assistance in identifying any errors and to let us know. Thank you for taking the time to report the errors by sending us an e-mail.
George A. Olah – Facts
George A. Olah – Biographical
 I was born in Budapest, Hungary, on May 22, 1927 the son of Julius Olah and Magda Krasznai. My father was a lawyer and to my best knowledge nobody in my family before had interest in science. I grew up between the two world wars and received a rather solid general education, the kind middle class children enjoyed in a country whose educational system had its roots dating back to the Austro-Hungarian Monarchy. I attended a Gymnasium (a combination of junior and senior high school) at one of the best schools in Budapest run by the Piarist Fathers, a Roman Catholic order. A strict and demanding curriculum heavily emphasizing the humanities included 8 years of Latin, with German and French as other obligatory languages. Although we had an outstanding science teacher who later became a professor of physics in the University of Budapest I can not recollect any particular interest in chemistry during my school years. My main interest was in the humanities, particularly history, literature, etc. I was (and still am) and avid reader and believe that getting attached too early to a specific field frequently shortchanges a balanced broad education. Although reading the classics in Latin in school may be not as fulfilling as it would be at a more mature age, few scientists can afford the time for such diversion later in life.
I was born in Budapest, Hungary, on May 22, 1927 the son of Julius Olah and Magda Krasznai. My father was a lawyer and to my best knowledge nobody in my family before had interest in science. I grew up between the two world wars and received a rather solid general education, the kind middle class children enjoyed in a country whose educational system had its roots dating back to the Austro-Hungarian Monarchy. I attended a Gymnasium (a combination of junior and senior high school) at one of the best schools in Budapest run by the Piarist Fathers, a Roman Catholic order. A strict and demanding curriculum heavily emphasizing the humanities included 8 years of Latin, with German and French as other obligatory languages. Although we had an outstanding science teacher who later became a professor of physics in the University of Budapest I can not recollect any particular interest in chemistry during my school years. My main interest was in the humanities, particularly history, literature, etc. I was (and still am) and avid reader and believe that getting attached too early to a specific field frequently shortchanges a balanced broad education. Although reading the classics in Latin in school may be not as fulfilling as it would be at a more mature age, few scientists can afford the time for such diversion later in life.
After graduating from high school and having survived the ravages of war in Budapest and realizing the difficulties facing life in a small and war torn country, I started to study chemistry upon entering university, being attracted by the wide diversity it offered.
Classes at the Technical University of Budapest were relatively small. We probably started with a class of 70 or 80, whose numbers were rapidly pared down during the first year to maybe half by rather demanding “do or die” oral examinations, where the ones who failed could not continue. This was a rather cruel process, because laboratory facilities were so limited that only few could be accommodated. At the same time the laboratory training was thorough. For example, in the organic laboratory we did some 40 Gatterman preparations. It certainly gave a solid foundation.
Organic chemistry particularly intrigued me and I was fortunate later to become a research assistant to Professor Geza Zemplen, the senior professor of organic chemistry in Hungary, who himself was a student of Emil Fischer in Berlin. He established in Hungary a reputable school in organic chemistry. As Fischer, he too expected his students to pay their own way and even paying for the privilege to work in his laboratory. Becoming an assistant to him although meant no remuneration but also no fee. Zemplen had a formidable reputation, and working for him was quite an experience. He also liked partying and these remarkable events in neighboring pubs lasted frequently for days. Certainly one’s stamina developed through these experiences.
Zemplen was a carbohydrate chemist, much interested in glycosides. Early in our association it became clear that my ideas and interest were not always closely matching his. When I suggested that fluorine containing carbohydrates may be of interest in coupling reactions, his reaction was not unexpectedly very negative. To try to pursue fluorine chemistry in post-war Hungary was indeed far fetched. Eventually, however, he gave in. Even basic chemicals needed for the work, such as HF, FSO3H or BF3 were non-existent and I made them myself, with enthusiastic help by some of my early associates (A. Pavlath, S. Kuhn). Laboratory space, particularly hoods (the kind exhausted only by draft caused by a gas burner causing warm air to raise and take some of the obnoxious fumes through a chimney) was very scarce and even by the time I became an assistant professor it was not welcome to “pollute” more important conventional work. However, the Institute which was on the second floor of the chemistry building, had in the back an open balcony, used to store chemicals. In one of his unexpected gestures Zemplen agreed that I can have the use of this balcony. With some effort we enclosed it, installed two old hoods and were soon in business in what was referred to as the “balcony laboratory”. I am not sure that Zemplen even set foot in it. We enjoyed, however, our new quaters and the implicit understanding that our fluorine chemistry and related study of Friedel-Crafts reactions and their intermediates was now officially tolerated.
Some of my publications in the early 50s from Hungary caught the eye of Hans Meerwein. It is still a mystery to me how he came to read them in a Hungrian journal, although there also was a foreign language edition of the Hungarian Chimica Acta. Anyhow, I received an encouraging letter from him and we followed up correspondence (not easy at a time in completely isolated Hungary). He must have sympathized with my difficulties because one day through his efforts I received a cylinder of boron trifluoride. What a precious gift it was!
The Hungarian educational system after the Communist takeover was realigned according to the Soviet example. University research was deemphasized and research institutes were established under the auspices of the Academy of Sciences. I was invited to join the newly established Central Chemical Research Institute of the Hungarian Academy of Sciences in 1954 and was able to establish a small research group in organic chemistry, housed in temporary laboratories of an industrial research institute. With my group, which by now also included my wife, we were able to expand our work and made the best of our possibilities. In October 1956 Hungary revolted against the Soviet rule, but the uprising was soon put down by drastic measures and much loss of life. Budapest was again devastated and the future looked rather dim. In November-December 1956 some 200,000 Hungarians, mostly of the younger generation fled their country. With my family and much of my research group we also decided to follow this path and look for a new life in the West.
I married in 1949 Judith Lengyel, the best thing ever to happen to me in my life. We knew each other from our early youth and are happily married now for more than 45 years. Judy worked initially as a technical secretary at the Technical University. After we were married she enrolled to study chemistry. She probably rightly recalls that I was entirely responsible for this step and she only agreed to get along with her single minded husband who seemed to believe that there is little in life outside chemistry. From my point of view for husband and wife to closely understand each other’s work and may even work together was most desirable. Our older son George John was born in Budapest in 1954. After we fled Hungary in early December of 1956, we reached late in December London where my wife had relatives. We subsequently moved on in the spring of 1957 to Canada, where my mother-in-law lived in Montreal after the war. During our stay in London for the first time I was able to establish personal contact with some of the organic chemists, whose work I knew and admired from the literature. I found them most gracious and helpful. In particular Christopher Ingold and Alexander Todd extended efforts on behalf of a young, practically unknown Hungarian refugee chemist in a way which I never forget and for which I am always grateful.
Dow Chemical, with its home base at Midland, Michigan was establishing at the time a small exploratory research laboratory 100 miles across the border in Sarnia, Ontario where its Canadian Subsidaries major operations were located. I was offered a position to join this new laboratory and they also hired two of my original Hungarian Collaborators, including Steven Kuhn. We moved to Sarnia in late May of 1957. As our moving expenses where paid we checked in two cardboard boxes containing all of our worldly possessions unto the train from Montreal and started our new life. Our younger son Ronald Peter was born in Sarnia in 1959. There was no possibility for Judy to continue her career at the time. Sacrificing her own career she devoted herself to bring up our children. She rejoined in our research only a decade later in Cleveland after I returned to academic life.
The Sarnia years at Dow were productive. It was during this period in the late 50’s that my initial work on stable carbocations was started. Dow was and is a major user of carbocationic chemistry, such as the Friedel-Crafts type manufacture of etylbenzene for styrene production. My work thus also had practical significance and helped to improve some industrial processes. In return I was treated well and given substantial freedom to pursue my own ideas. Eventually I was promoted to company Scientist, the highest research position without administrative responsibility.
In the spring of ’64 I transferred to Dow’s Eastern Research Laboratories in Framingham, Massachusetts established under Fred McLarrerty’s directorship. The laboratory was subsequently moved to Wayland, just outside Boston. In the summer of 1965 I was invited to join Western Reserve University in Cleveland, Ohio and returned to academic life as professor with the added responsibility of becoming also Department Chairman.
My Cleveland years were both scientifically and personally most rewarding. My wife Judy was able to rejoin me in our research and my research group grew rapidly. The chemistry departments of Western Reserve University and neighboring Case Institute of Technology were practically adjacent, separated only by a parking lot. It became obvious that it would make sense to join the two into a single, stronger department. We achieved this by 1967 with surprisingly little friction and I was asked to serve as the Chair of the joint department till things settled down. It was in 1969 that I was able to give up my administrative responsibility. As I worked hard my research never suffered during this period and as a matter of fact these were probably some of my most productive years.
After 12 years in Cleveland it was time again to move on. Our older son George was approaching the end of his college years and our younger son Ron who was finishing high school set his mind to go to Stanford. He convinced us that it should be nice for the whole family to resettle in California. Coincidentally, in the fall of 1976 Sid Benson, an old friend called me to find out whether I would be interested to join him at the University of Southern California in Los Angeles. After some visits to LA the challenge of trying to build up chemistry in a dynamic university and the attractiveness of life in Southern California convinced us to move. We fell in love with California and we still are. As USC had limited chemistry facilities, it was offered to establish a research institute in the broad area of hydrocarbon research and provide it with its own building and facilities. We moved in May of 1977. Some 15 members of my research group joined the move West. By arrangements worked out we were able to take with us most of the laboratory equipment, chemicals, etc. Two weeks after our arrival with some large moving vans we were back doing chemistry in temporary quarters, while our research institute was constructed. The Institute was established at USC with generous support by Mr. & Mrs. D.P. Loker, friends and great supporters of the University. The Institute was subsequently named after them. Don Loker passed away some years ago, but Katherine still chairs the Institute’s board. Through her and other friends’ generosity a wonderful new addition to our Institute is just completed doubling our space.
As rewarding as the Nobel Prize is personally to any scientist, I feel it is also recognition of all my past and present students and associates (by now numbering close to 200), who contributed over the years so much through their dedicated hard work to our joint effort. It also recognizes fundamental contributions by many colleagues and friends from around the world to a field of chemistry, which is not frequently highlighted or recognized.
This autobiography/biography was written at the time of the award and later published in the book series Les Prix Nobel/ Nobel Lectures/The Nobel Prizes. The information is sometimes updated with an addendum submitted by the Laureate.
Addendum, May 2005
It is frequently said that receiving the Nobel Prize put so many obligations and commitments on the winners that their scientific work inevitably suffers. I was much determined that this should not happen to me. Having received the prize at the age 67 also helped, as my lifelong habits were solidly developed. I was determined that the prize should not affect significantly my life and certainly not my research.
I feel that I mostly succeeded. The intervening years were very productive and in many ways most rewarding for my research. Helped by my dedicated younger colleagues and associates and by close collaboration with my colleague Professor Surya Prakash, I was able to not only to continue my research but to extend it into new challenging areas.
A significant part of my previous research was based on the study of positively charged carbon compounds (carbocations) using superacids and their chemistry. The extremely strong acids I used and explored turned out to be many billions or even trillions of times stronger than previously recognized “strong” acids such as concentrated sulfuric acid.
The vastly increased acidity of superacidic systems resulted in the significant new field of superacid chemistry. In the last decade I asked myself whether a similar but more general approach could be used to produce in general electrophiles (electro deficient reagents) of greatly enhanced electron reactivity.
This resulted in the development of the concept of superelectrophilic activation and the study of superelectrophiles, i.e. electrophiles of greatly enhanced reactivity compared with previously known related electrophilic reagents and systems.
The concept of superelectrophiles thus emerged from my previous studies on superacidic carbocation and onium ion systems. It is based on the realization that a variety of electrophiles capable of further interaction (coordination) with strong Bronsted or Lewis acids can be greatly activated by them. Examples include onium and carboxonium ions, acyl cations, halonium, azonium, carbozonium ions, even certain substituted carbocations and the like. This activation produces what is now known as superelectrophiles, that is, electrophiles of doubly electron-deficient (dipositive) nature whose reactivity significantly exceeds that of their parents. Superelectrophiles are the de facto reactive intermediates of many electrophilic reactions in superacidic systems (including those involving solid superacids) and even some enzymatic systems and should be differentiated from energetically lower-lying, thus much more stable intermediates, which frequently are observable and even isolable but are not necessarily reactive enough without further activation.
Examples of some superelectrophiles so far studied and their parents are
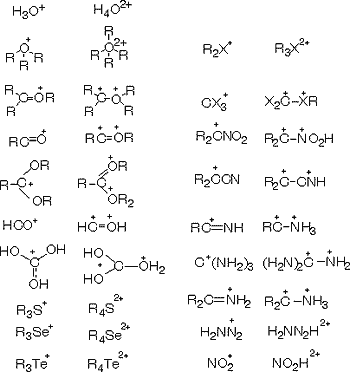
It should be recognized that superelectrophilic reactions frequently proceed with only “electrophilic assistance” (solvation, association) by the superacids without forming distinct dipositive intermediates. Protosolvolytic activation of electrophiles should always be considered in this context.
Another area of my post-Nobel research, that turned into a major continuing effort, evolved from the realization that our hydrocarbon resources, the marvelous gift of nature in the form of petroleum oil, natural gas and coal, are finite and not renewable.
The rapidly growing world population, which was 1.6 billion at the beginning of the twentieth century, has now well exceeded 6 billion. Even if mankind increasingly would exercise population control, by mid-century we will reach around 9.5-10 billion. This inevitably puts enormous pressure on our resources, not the least on our energy resources. For its survival, mankind needs not only food, clean water, shelter clothing, etc. but also energy. Since the cave man first managed to keep light and fire, our early ancestors burned wood and subsequently other natural sources. The industrial revolution was fueled by coal. The twentieth century added oil and natural gas and introduced atomic energy.
When fossil fuels such as coal, oil, or natural gas (i.e. hydrocarbons) are burned in power plants to generate electricity or to heat our homes and fuel our cars and airplanes, they form carbon dioxide and water. Thus, they are used up and are nonrenewable (at least on the human time scale). To find ways to replace our diminishing natural resources hydrocarbons will to be made by ourselves in a renewable, economical, and environmentally adaptable, clean way. This represents a major challenge for mankind in the twenty-first century.
I have developed a promising new approach for solving not only our long range dependence on decreasing fossil fuels (oil, gas, and coal) but also at the same time to mitigate global climate change (warming) caused significantly by derived greenhouse gases such as carbon dioxide and methane. The approach is based on the use of methanol (CH3OH) as a way to store energy, as well as a convenient fuel and hydrocarbon source. Methanol as a fuel can also be directly used in the new fuel cell we developed jointly with the Jet Propulsion Laboratory of Caltech. It is also a raw material for synthetic (man made) hydrocarbons through its conversion to ethylene or propylene (by catalytic bimolecular dehydration i.e. 2CH3OH->CH2=CH2+2H2O). From these one can produce all the hydrocarbon fuels and products (from gasoline and diesel oil, to plastics, synthetic materials, pharmaceuticals, etc.), which are currently made from oil and natural gas. I call this new approach the “methanol economy”.
Currently methanol is still produced from fossil fuels, predominantly from natural gas through syn-gas (a mixture of CO and H2) by so-called Fisher-Tropsch chemistry, which is however a highly energy vasting process. We have developed new methods to convert still existing natural gas (methane) directly and efficiently to methanol. The true methanol economy, however, will do without natural gas, oil and coal as it is possible to produce methanol by the reaction of carbon dioxide with hydrogen. Exhaust gases from power plants and varied industrial emissions contain considerable amounts of carbon dioxide, which can easily be separated. Rather than just collecting carbon dioxide and storing it underground or at the bottom of the seas (as it is suggested) it can be used to produce methanol. Eventually, atmospheric carbon dioxide itself will be possible to be separated and converted to methanol. As atmospheric carbon dioxide is available to all people on the Earth this will enable mankind to liberate itself from dependence on fossil fuels. Substantial energy is of course necessary to generate the needed hydrogen for methanol production. This energy could come from safe nuclear power plants as well as all alternative energy sources such as sunlight, wind, geothermal, etc. At the same time, this approach will also diminish the danger of global warming by removing and recycling the rising carbon dioxide content of the atmosphere.
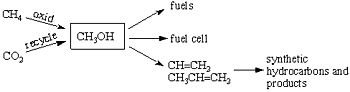 |
I am fortunate to have retained my interest and drive to continue research with quite unabated energy. I published in the last decade years a number of books and monographs, notably: “Hydrocarbon Chemistry” (with Arpad Molnar,) 2nd revised ed., Wiley, 2003. “Onium Ions” (with Kenneth Laali, Qi Wang, and Surya Prakash,) Wiley, 1998. “Across Conventional Lines, selected papers of George Olah” (ed. with Surya Prakash), World Scientific Publishing, Singapore, 2003. “Carbocation Chemistry” (ed. with Surya Prakash), Wiley, 2004. “Beyond Oil and Gas: The Methanol Economy” (with Alain Goeppert and Surya Prakash), Wiley-VCH, 2005 (in preparation). I also published about 170 additional research papers and obtained some 15 patents.
For more biographical information, see:
Olah, George A., A Life Of Magic Chemistry: Autobiographical Reflections of a Nobel Prize Winner. Wiley-Interscience, New York, 2000.
George A. Olah died on 8 March 2017.
Copyright © The Nobel Foundation 2005
Hydrocarbons for the 21st century

Hydrocarbons for the 21st century – The work of the Loker Hydrocarbon Research Institute
by George A. Olah
1994 Nobel Prize laureate in chemistry
This article was published on 6 September 1999.
Hydrocarbons derived from petroleum, natural gas, or coal are essential in many ways to modern life and its quality. The bulk of the world’s hydrocarbons is used for fuels, electrical power generation, and heating. The chemical, petrochemical, plastics and rubber industries are also dependent upon hydrocarbons as raw materials for their products. Indeed, most industrially significant synthetic chemicals are derived from petroleum sources. The overall oil use of the world now exceeds ten million metric tons a day. Ever increasing world population (about 6 billion to increase to 10 billion in a few decades) and energy consumption and finite non-renewable fossil fuel resources, which are going to be increasingly depleted, are clearly on a collision course. New solutions will be needed for the 21st century if we are to maintain the standard of living the industrialized world has gotten used to and the developing world is striving to achieve.
Recognizing the need for a long-range program of basic research and graduate education in the field of hydrocarbon chemistry, the University of Southern California established its “Loker Hydrocarbon Research Institute”1 in 1977. Generous donations from Donald and Katherine Loker, as well as other friends and supporters helped build an outstanding facility and program.
Hydrocarbon chemistry
Hydrocarbons, the principal compounds of oil and natural gas, have to be chemically altered to make useful products and materials. This is carried out by chemical and petrochemical industries in processes such as isomerization, alkylation homologation, etc. These processes are frequently catalyzed by acids and involve electron deficient intermediates called carbocations. The Loker Institute has pioneered new methods to study such processes and their mechanisms. Research is also aimed at more efficient utilization of fossil fuel resources including recycling of carbon dioxide (a greenhouse gas) to useful materials. Studies are also directed towards developing new synthetic methodologies for chemical bond making and bond breaking processes. Polymeric materials derived from simple hydrocarbon precursors are the basis for new materials with exceptional electrical, optical, and magnetic properties. These materials find applications in information technology, photochemical energy conversion and biomedical devices.
Carbocarbons and their chemistry
In studying hydrocarbons and their conversions, a wide variety of highly acidic systems called superacids have been developed. When higher valent Lewis acid fluorides such as SbF5 and TaF5 are combined with Brönsted acids such as HF or FSO3H, acids many billions of times stronger than sulfuric acid are obtained. In such superacidic media the lifetime of carbocations are sufficiently long to be examined by a variety of chemical and physical methods including nuclear magnetic resonance spectrometry.
Tertiary Butyl Cation
Copyright © Loker Hydrocarbon Research Institute
Acid catalyzed conversion of hydrocarbons such as cracking, isomerization, alkylation, oligo- and poly-condensation, etc. are of substantial importance. The fundamental chemistry of such hydrocarbon conversions involves carbocations and their reactions. Novel environmentally benign acid systems, including solid acids, are developed to overcome difficulties connected with toxic acids such as hydrofluoric or sulfuric acid. Isomerization and alkylation of saturated hydrocarbons to provide high octane gasoline are of particularly great importance in the petroleum industry. The Loker Institute has developed an environmentally friendly and practical alkylation process for the manufacture of high octane gasoline by using a modified hydrogen fluoride catalyst system of greatly reduced volatility and toxicity.

Dr. Olah preparing t-Butyl Cation.
Photo by Dr. Herwig Buchholz
In addition, the use of superacidic catalysts allow new ways to hydro-treat coals, shale oil, tar sands and other heavy petroleum sources and residues, and yield liquid hydrocarbons. New and environmentally safe gasoline and diesel fuel additives were also developed, resulting in higher octane gasoline and higher octane diesel fuels. These additives have also resulted in cleaner burning fuels and opened the way to exclude currently used other toxic additives.
Conversion of methane or carbon dioxide to hydrocarbons
The direct conversion of methane (i.e. natural gas) to higher hydrocarbons and derived products offers a viable alternative to Fischer-Tropsch chemistry (utilizing synthesis gas, i.e. CO and H2). Until recently, the utilization of methane as a chemical building block was limited to free radical reactions (combustion, nitration, chlorination, etc.). Various stoichiometric organometallic insertion reactions were also discovered, but their use is so far not practical. Superacid catalysts developed at the Institute permit oxidative condensation of methane to higher hydrocarbons, as well as the selective electrophilic conversion of methane to its mono-substituted derivatives such as methyl halides and methyl alcohol. Monosubstituted methanes can be further condensed to ethylene, propylene and derived hydrocarbons over zeolites or bifunctional acidic-basic catalysts, giving access to a whole range of hydrocarbons essential to our everyday life.
Methonium ion
Copyright © Loker Hyrdocarbon Research Institute
Mechanistic aspects of the methane conversion chemistry, particularly the role of pentacoordinate CH5+-type carbocationic intermediates, were also studied. Kekule’s conclusion dating back to the 1860’s that carbon cannot bound to more than four atoms of groups, i.e. it cannot exceed tetravalency, was refuted by discoveries obtained at the Institute. Dr. Olah’s substantial body of work in this area resulted in the realization that in electrodeficient (carbocationic) systems carbon can coordinate with five, six or even seven atoms or groups simultaneously and laid the foundation to what is now recognized as hypercarbon chemistry.
When hydrocarbons are burned they form carbon dioxide and water. They are thus non-renewable on the human time scale. Excessive burning of fossil fuels leads to increased atmospheric levels of carbon dioxide, which has been linked to global warming and climatic changes. In addition to trying to keep carbon dioxide levels down through reducing burning of fossil fuels (the basis of the 1997 Kyoto agreement), new solutions are needed. An innovative new approach pursued by the Institute is directed at reversing the process by producing hydrocarbons from carbon dioxide and water via methyl alcohol. Some of the underlying chemistry to convert carbon dioxide using hydrogen gas (obtained by electrolytically splitting water) is known. Metal or superacid catalyzed reduction pursued by the Institute has made significant progress to bring about the feasibility of CO2 conversion to methanol. However, electricity needed for generating hydrogen is costly and remains the key to practical applications. As we still cannot store electricity efficiently, power plants in their off-peak periods could produce hydrogen as a means of storing electricity. Hydrogen then could be used to recycle CO2 (from smokestack emissions or other concentrated sources, eventually even the atmosphere) into methyl alcohol and derived fuels. The carbon dioxide recycling technology now under development allows us not only to produce useful fuels and hydrocarbon products, at the same time can contribute to mitigating CO2 related global warming.
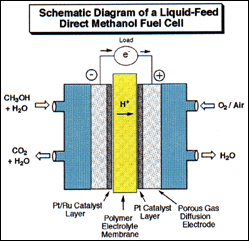
Diagram by Dr. G. K. Surya Prakash
Methyl alcohol and derived fuels can also be used to produce electricity in the new direct oxidation liquid feed fuel cells developed jointly by the Loker Institute and Caltech-JPL. When operating the fuel cell in its “reversed mode”, carbon dioxide and water can be electro-catalytically reduced to methyl alcohol. While the recycling of carbon dioxide into hydrocarbons is a highly energy demanding process some applications, i.e. solar power related applications, may not be overly concerned with this high energy input requirement.
Even if technologies to generate energy from alternate sources are further developed (i.e. atomic, solar, wind, etc.), a concentrated research effort is required to find long-range solutions for future hydrocarbon needs. The effort must include the development of alternative hydrocarbon sources, a search for new chemistry directed towards exploitation of renewable fuels, as well as the development of more efficient and environmentally acceptable ways of utilizing and recycling our present resources.
The final solution to the shortage of hydrocarbons will come only when mankind can produce cheap energy through safer atomic energy (or even fusion) and other alternate sources. With abundant cheap energy, hydrocarbons will be produced from carbon dioxide of the atmosphere and water. In the meantime, however, it is essential that solutions be found that are feasible within the framework of our existing technological base.
Notes
1. The Loker Hydrocarbon Research Institute

The Loker Hydrocarbon building (east elevation)
Photo: Adrian Velicescu
Located at the heart of the University of Southern California campus in Los Angeles, California, the Loker Institute’s 43,000 square feet building features well-equipped state-of-the art laboratories and an attractive work environment. The George and Judy Olah library and the splendid reading room atop the building greatly facilitate the research and educational efforts. The Institute’s plant and equipment represents an investment in excess of twenty-five million dollars. These assets were provided through private donations. At any time about 60 researchers work in the Institute. Their work is ably-supported by a small, but dedicated administrative and technical staff. Financial support for the Institute’s work comes from an annual budget and salaries appropriated by the University, research grants and contracts, income from endowment funds, gifts and donations, as well as income from patents of Institute scientists.
There are four endowed professorships associated with the Institute. The overall annual operational cost of the Institute amounts to about five million dollars. To assure the long-range stability of the Institute, our friends and supporters established endowments, which are augmented continuously. Income from these endowments supplement operating funds, and allows the Institute to carry out exploratory pioneering research. It also makes possible visits and lectures by outstanding national and international scholars and supports scientific symposia hosted by the Institute. An advisory board chaired by Mrs. Katherine Loker, oversees the work at the Institute.

Dr. Olah receiving the 1994 Nobel Prize in Chemistry
© Pressens Bild AB. Photo: Jan Collsiöö
Scientific and Educational Goals
The Loker Institute’s goal is to further fundamental research and advanced training in the broad area of hydrocarbon research. Its work has been guided since its inception by its director, Nobel Laureate Professor George A. Olah.
To date, the Institute’s scientific work has resulted in over 1000 refereed publications in leading technical journals and more than a dozen monographs and books dealing with fundamental research on hydrocarbons. Scores of patents have been issued based on discoveries at the Institute, some of which resulted in industrial processes (vide infra).
A growing need for research in the area of hydrocarbon chemistry also implies an increased demand for chemists who are trained in this field of science. An essential part of the Loker Institute’s mission is to train future generations of scientists by creating and maintaining an educational environment which fosters innovative and practical research in the advanced chemistry of hydrocarbons while helping individuals realize their scientific and academic potential. Since its inception, the Institute has trained more than 300 Ph.D. and post-doctoral fellows who have come from all corners of the world to the Institute. Graduates of the Institute have excelled in both industry and academia. Their success is a testimony to the Institute’s efforts.
First published 6 September 1999
Press release

12 October 1994
The Royal Swedish Academy of Sciences has decided to award the 1994 Nobel Prize in Chemistry to
Professor George A. Olah, University of Southern California, USA
for his contributions to carbocation chemistry.
Carbocations: from hypothetical intermediate products to well defined molecules
Most of us can recall from our high school chemistry courses that many so called inorganic compounds, like for example ordinary table salt, NaCl, could be regarded as built up from atoms or groups of atoms that are electrically charged. This table salt can be regarded as positively charged sodium ions (Na+) forming a bond with negatively charged chlorine ions (Cl–). To take another example; “glauber salt”, Na2SO4, can be thought of as two Na+ ions forming a bond with one SO42- ion.
While such electrically charged species – “ions” – are common in the world of inorganic compounds the opposite is true in the world of organic compounds, particularly in the case of the so called hydrocarbons. Hydrocarbons are compounds that are made up from only two types of elements – hydrogen (H) and carbon (C). Hydrocarbons constitute a very large and important group of organic compounds – for example most products from natural mineral oil are hydrocarbons. Although some hydrocarbons prepared by chemists around the turn of the century were thought to be ionic – e.g. a group of compounds formed from benzene and methane (“triarylmethane derivatives”) these were largely regarded as curiosities.
When some chemists in Britain (Ingold & Hughes) and Germany (Meerwein) in the 1920s and 1930s started detailed studies of how chemical reactions between organic molecules took place it, however, became apparent that positively charged hydrocarbons – what chemists call “carbocations” – actually could occur as very short lived (lifetimes of microseconds to nanoseconds) intermediates in the reactions.
Since these postulated “carbocation intermediates” were likely to be not only very short lived but also very reactive, it was generally assumed that one would never be able to prepare them in some quantities. Nor be able to study their properties with different physical techniques – e.g. NMR and infrared (IR) spectroscopy or X-ray diffraction – like one could do with normal uncharged hydrocarbons. But the direction of this field did change completely through the original and imaginative work by this years Nobel Prize laureate in Chemistry Professor George A. Olah.
In the early 1960s Olah and co-workers discovered that stable carbocations could be prepared through the use of a new type of extremely acid compounds – far stronger than “classical” acids like sulphuric acid, hydrochloric acid etc. These new acids – some of which were first described by the Canadian inorganic chemist, R. J. Gillespie – became generally known as “superacids”. A superacid can for example be prepared from hydrogen fluoride (HF) and antimony pentafluoride (SbF5).
Olah’s discovery completely transformed the scientific study of the elusive carbocations. Since the original discovery a large number of carbocations have been prepared and their properties studied in great detail. Olah has also shown how basic knowledge on superacids and carbocations can be applied to the facile synthesis of new and important organic compounds and that a number of small organic molecules, with widespread use as starting material in many large scale synthesis, can be produced in a simple and inexpensive way using superacids as catalysts. His work has resulted in new methods for the conversion of straight chain hydrocarbons (when used in combustion engines these have very low octane number and they are also difficult to degrade biologically) into branched hydrocarbons that have high octane numbers and are more easily biodegradable.
Olah’s scientific contributions have won widespread recognition among organic chemists and his work on carbocations now has a prominent position in all modern textbooks on organic chemistry.
Background
George A. Olah has through his research on the cations from carbon compounds (carbocations) in superacidic solvents and at low temperatures opened new avenues towards new and detailed knowledge of their structure and reactivity. His work has also led to the discovery of new reactions of considerable potential in the chemical industry and elsewhere.
In a chemical reaction, the molecules of the original material are converted into a final product. This most often occurs via very short-lived (10-10 – 10-6seconds) entities termed reactive intermediates. In many organic reactions these are carbocations. Since they are so short-lived they occur in such low concentrations that they cannot be directly observed with, for example, spectroscopy. Knowledge of their existence, structure, reactivity and so on has therefore been very incomplete.
It is important to understand how reactions proceed to be able to control them, intervening to obtain the products desired. This is especially important for the chemical industry. In his research, Olah endeavoured to give the short-lived carbocations a long life. It was necessary to get them to react more slowly with solvents and other nucleophilic molecules. (Nucleophiles are anions or solvents that have a free electron pair and that can attack a positive ion or a positively polarised atom in a molecule.) Olah found he could use solvents that were very little nucleophilic (e.g. S02, SO2ClF and SO2F2) and that therefore at low temperatures react slowly with carbocations. To produce carbocations he used what are termed superacids (acids that are stronger than 100% sulphuric acid).
Dissolving alkyl halides at low temperature in hydrogen fluoride-antimon pentafluoride (HF-SbF5), which is 1018 times stronger than 100% sulphuric acid, he managed for the first time to produce trivalent carbocations (carbenium ions) in such high concentrations and of such long life spans that, using nuclear magnetic resonance spectroscopy (NMR) and electron spectroscopy for chemical analysis (ESCA), he was able to study their structure, stability, properties and reactivity. Olah’s pioneering work has made it possible to observe carbocations directly with various spectroscopic methods and to gain detailed knowledge of their structure and reactivity.
Olah also found that superacids are so strong that they can even bind more hydrogen ions to simple hydrocarbons, forming pentacoordinated carbocations (carbonium ions). This has already had practical consequences in hydrocarbon chemistry, leading, for example, to new methods of isomerising hydrocarbons and synthesising higher hydrocarbons from methane.
Very many carbocations of great structural variation have now been studied. The results have brought many surprising and important contributions to our understanding of chemical bindings. Besides trivalent (tricoordinated) carbocations, carbocations with higher coordination – tetra, penta- and hexacoordination – have been generated and their structure determined. The old dogma of the tetravalency of carbon, a cornerstone of structural organic chemistry since the days of Kekulé in the 1860s, was thus destroyed.
History
During the 1920s, mainly through the research of C.-K Ingold (1893-1970, UK), the mechanisms of many organic reactions were elucidated. Two of the commonest and most widely used reactions in synthetic organic chemistry are nucleophilic substitution and elimination. In nucleophilic substitution the attacking reagent (the nucleophile) carries an electron pair to the substrate, using this pair to form the new bond while the leaving group departs with an electron pair. In elimination, two groups on adjacent carbon atoms are lost and an olefine (alkene) is formed. Depending upon the structure of the substrate, the solvent and a number of other factors, these reactions can occur in two stages. These are exemplified below with isopropyl chloride which, in the presence of the nucleophile Nu-, reacts to give a nucleophilic substitution product and/or an elimination product:
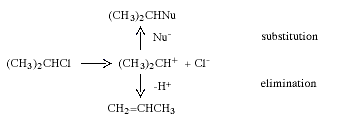
In the first stage the carbon-chlorine bond is split, and a short-lived carbenium ion is obtained. In the next step this rapidly reacts either with Nu- and gives the substitution product or transfers a proton to the solvent or Nu- to give propene.
The results of extensive kinetic and stereochemical investigations were consistent with mechanisms involving carbocations as intermediates. Carbenium ion structures and carbonium ion structures (non-classical ions) were suggested as hypothetical intermediates. To explain the results, it was also necessary to assume that the carbocations could often be associated with some negatively charged ion to give contact-ion pairs or solvent-separated ion pairs. A prominent figure in this later research was S. Winstein (1912-1969, USA).
The carbocations studied, however, were so short-lived (10-10– 10-6 seconds) that they could not be directly observed with spectroscopy. The picture of these important intermediates still remained incomplete.
Olah’s extremely important contribution lies in the methods he evolved for developing carbocations in high concentrations and under conditions which give them long life. To achieve this, he used solvents which were so extremely little nucleophilic that they did not attack carbocations. Such solvents are SO2, SO2ClF and SO2F2in which, at least at temperatures around -100°C, carbocations do have long life. To generate carbocations, Olah used various superacids including SbF5, which is a Lewis superacid, giving carbocations with e.g. alkyl halides. Others were Brønsted superacids such as HSO3F or the extremely strong superacids obtained by combining e.g. HSO3F or HF with SbF5. Magic Acid®, HSO3F:SbF5 and HF:SbF5 are 1018 times stronger than 100% sulphuric acid. Of these, Magic Acid® and H:SbF5 are so strong that they can completely protonate alcohols and olefines, thus giving carbocations in high concentrations. Temperatures between -78°C and -120°C are usually used. Especially 1H- and 13C- NMR-spectroscopic studies have given detailed knowledge of the structure, stability and reactivity of carbocations.
Investigations of the nucleophilic substitution reactions and rearrangements of 2-norbornyl derivatives led S.Winstein to suggest in the early 1950s that the intermediate carbocation was non-classical and contained a pentacoordinated carbon (C6 in Figure la coordinates to two hydrogen atoms besides C1, C2 and C5). This interpretation was questioned by H.C. Brown (1912, USA), who received the 1979 Nobel Prize in Chemistry for his development of boron compounds into important reagents in organic synthesis. Brown claimed that the 2-norbornyl cation did not have carbonium ion structure but was a carbenium ion that rapidly rearranged itself into itself, i.e. it was a rapidly equilibrating carbenium ion (Figure 1b).
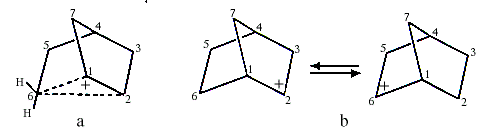 |
| Figure 1. a) Carbonium ion b) Equilibrating carbenium ions |
The ensuing scientific controversy lasted until about 1980. As the structures of carbonium ions were of great theoretical interest, the problem fascinated many leading physical organic chemists, yet despite great efforts and many ingenious experiments, no definitive solution was found until the 2-norbornyl cation could be directly studied with NMR-spectroscopy.
Olah and his co-workers finally observed the 2-norbornyl carbocation in a solution of SbF5-SO2ClF-SO2F2 at -158°C. Both 1,2-hydride shifts and more complicated rearrangements at this low temperature are slow enough not to disturb the interpretation of the NMR-spectra.
The spectra accorded completely with Winstein’s symmetrical bridged structure, with a pentacoordinated carbon (Figure la) and not with a rapidly equilibrating carbenium ion. Studies using electron-spectroscopy for chemical analysis (ESCA), developed by the Swedish Nobel laureate in physics for 1981, Kai Siegbahn, confirmed these conclusions.
Molecules containing pentacoordinated carbon atoms are no longer an exotic curiosity in organic chemistry. They have been found in inorganic compounds, organometallic compounds e.g. organolithium compounds, carboranes and other cluster compounds.
Superacids are so strong that they can protonate such extremely weak bases as the alkanes, as was shown by Olah and independently by H. Hogeveen. Thus, pentacoordinated carbonium ions have been obtained from methane higher alkanes and various cycloalkanes. Methane gives the methionium ion CH5+, which Olah has formulated as containing a three-centre, two-electron bond (Figure 2a, indicated with triangular dotted lines). Note that their junction does not represent an additional atom. Higher alkanes can be protonated both at C-H bonds (Figure 2b) and at C-C bonds (Figure 2c).
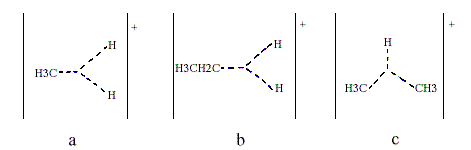
Olah found that for instance protonated isobutane decomposes to the t-butyl cation and molecular hydrogen. From the classical point of view, this is quite an unreasonable reaction; isobutane is oxidized by the proton to give the t-butyl cation and molecular hydrogen.
The protonation of saturated hydrocarbons in superacidic media has, through Olah’s work, already had practical consequences. It has led to a method for isomerising straight alkanes into branched alkanes of higher octane number. It has permitted the preparation of higher alkanes with methane as building block, illustrated below in the formation of ethane from methane. Superacid catalysis has also made it possible to crack heavy oils and to liquefy coal under surprisingly mild conditions.
![]()
Olah has recently shown that our most common electrophiles such as the acyl cation and the nitronium ion are protonated in superacidic media into doubly charged superelectrophiles. This leads to a dramatic increase in electrophilic reactivity. It is already obvious that Olah’s new chemistry has very broad and important applications.