Nobelpriset i kemi 2004 – Populärvetenskaplig information
English
Swedish

Populärvetenskaplig information
6 oktober 2004
En mänsklig cell innehåller hundratusentals olika proteiner. Dessa har en rad olika och viktiga funktioner: som påskyndare av kemiska reaktioner i form av enzymer, som signalsubstanser i form av hormoner, som viktiga aktörer i immunförsvaret i form av antikroppar och genom att stå för form och struktur i cellen. Årets pristagare i kemi, Aaron Ciechanover, Avram Hershko och Irwin Rose, har bidragit med grundläggande kemisk kunskap om hur cellen kan reglera förekomsten av ett visst protein genom att märka ut oönskade proteiner med en etikett bestående av polypeptiden ubiquitin. Märkta proteiner bryts sedan snabbt ner i cellulära “avfallskvarnar” som kallas proteasomer.
Genom upptäckten av detta reglersystem för proteiner har Aaron Ciechanover, Avram Hershko och Irwin Rose gjort det möjligt att på molekylär nivå förstå hur cellen kontrollerar en rad mycket viktiga biokemiska processer såsom cellcykeln, reparation av DNA, transkription av gener samt kvalitetskontroll av nytillverkade proteiner. Denna form av styrd proteindöd har också bidragit till att förklara hur immunförsvaret fungerar. Defekter i systemet kan leda till en rad olika sjukdomar inkluderande olika typer av cancer.
Dödsmärkning av proteiner
Nedbrytning kräver väl inte energi – eller?
Medan stor uppmärksamhet och mycket forskning ägnats åt att förstå hur cellen kontrollerar syntesen av ett visst protein – åtminstone fem olika Nobelpris har utdelats inom detta område – har motsatsen, dvs. nedbrytningen av proteiner, länge ansetts mindre viktig. Man kände tidigt till en rad enkla proteinnedbrytande enzymer, t.ex. trypsin, som i tunntarmen bryter ner proteiner i vår föda till aminosyror. Likaså studerade man länge en typ av cellorganeller, lysosomer, i vilka proteiner som cellen tagit upp utifrån bryts ner. Gemensamt för dessa processer är att de inte kräver energi för att fungera.
Experiment utförda redan på 1950-talet visade dock att nedbrytning av cellens egna proteiner kräver energi. Detta förbryllade länge forskarna och det är just denna paradox som ligger bakom årets Nobelpris i kemi: att det krävs energi för nedbrytningen av proteiner inuti cellerna medan övrig proteinnedbrytning sker utan energitillförsel. Ett första steg för att komma närmare en förklaring av den energiberoende proteinnedbrytningen togs av Goldberg och medarbetare som 1977 utarbetade ett cellfritt extrakt från omogna röda blodkroppar, retikylocyter, vilket katalyserar nedbrytningen av onormala proteiner på ett ATP-beroende sätt (ATP = adenosintrifosfat, cellens energivaluta).
Genom att använda ett sådant extrakt lyckades Aaron Ciechanover, Avram Hershko och Irwin Rose i en rad epokgörande biokemiska studier under sent 1970-tal och tidigt 1980-tal visa att proteinnedbrytningen i cellerna sker i en serie stegvisa reaktioner vilka resulterar i att de proteiner som ska förstöras märks med polypeptiden ubiquitin. Denna process gör det möjligt för cellen att med hög specificitet bryta ner oönskade proteiner. Det är alltså denna reglering som kräver energi. Till skillnad från reversibla proteinmodifieringar som t.ex. fosforylering (Nobelpris i fysiologi eller medicin 1992) är reglering genom polyubiquitinering ofta irreversibel eftersom målproteinet bryts ner. En stor del av arbetet utfördes under en serie sommarledigheter som Avram Hershko och Aaron Ciechanover från Technion (Israel Institute of Technology) tillbringade hos Irwin Rose vid Fox Chase Cancer Center i Philadelphia, USA.
Etiketten heter ubiquitin
Den molekyl som senare skulle visa sig vara själva den etikett som märker ut ett protein för nedbrytning isolerades redan 1975. Denna 76 aminosyror långa polypeptid isolerades från kalvbräss och antogs delta vid bildandet av vita blodkroppar. Eftersom man senare fann molekylen i en mängd olika vävnader och organismer – dock inte i bakterier – fick den namnet ubiquitin (som betyder allmänt förekommande).
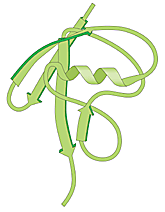 |
| Högupplöst bild (jpeg 184 kB) |
| Fig 1. Ubiquitin – en vanlig polypeptid i cellen som fungerar som själva “dödskyssen”. |
Upptäckten av ubiquitinmedierad proteinnedbrytning
Avram Hershko hade efter sin doktorsexamen studerat energiberoende proteinnedbrytning i leverceller, men beslöt 1977 att övergå till det ovan beskrivna retikylocytextraktet. Detta extrakt innehöll stora mängder hemoglobin vilket störde experimenten. I sina försök att med hjälp av kromatografi avlägsna hemoglobinet upptäckte Aaron Ciechanover och Avram Hershko att extraktet kunde delas upp i två fraktioner vilka var för sig var inaktiva. Men det visade sig att så fort man återförenade de två fraktionerna, satte den ATP-beroende proteinnedbrytningen igång igen. År 1978 rapporterade man att den aktiva komponenten i den ena fraktionen var en värmestabil polypeptid med en molekylvikt på endast 9 000 som man kallade APF-1 (= aktiv princip i fraktion 1). Detta protein visades senare vara just ubiquitin.
Det avgörande genombrottet i forskningen rapporterades i två arbeten som Ciechanover, Hershko och Rose publicerade 1980. Fram till dess var funktionen hos APF-1 helt okänd. I det första arbetet visade man att APF-1 bands kovalent, dvs. med en mycket stabil kemisk bindning, till olika proteiner i extraktet. I det andra arbetet visade man vidare att flera APF-1 molekyler kunde bindas till samma målprotein; man kallade det senare fenomenet polyubuquitinering. Vi vet nu att just polyubiquitinering av substratproteiner är den utlösande signal som leder till proteinets nedbrytning i proteasomen. Det är denna reaktion som utgör själva märkningen, “dödskyssen” om man så vill.
Dessa helt oväntade upptäckter ändrade i ett slag förutsättningarna för det fortsatta arbetet; nu kunde man koncentrera sig på att identifiera det enzymsystem som binder samman ubiquitin med dess målproteiner. Eftersom ubiquitin är så allmänt förekommande i olika vävnader och organismer insåg man snabbt att ubiquitinmedierad proteinnedbrytning måste ha en generell betydelse för cellen. Vidare gissade man att energibehovet i form av ATP ger möjlighet för cellen att kontrollera processens specificitet.
Fältet låg nu öppet och under perioden 1981–1983 utarbetade Ciechanover, Hershko, Rose tillsammans med deras post docs och studenter “the multistep ubiquitin-tagging hypothesis” baserad på tre nyupptäckta enzymaktiviteter som man kallade E1, E2 och E3 (fig. 2). I dag vet vi att en typisk däggdjurscell innehåller ett eller ett par olika E1-enzym, något fler E2-enzym och flera hundra olika E3-enzym. Det är E3-enzymens specificitet som avgör vilka proteiner i cellen som ska märkas för destruktion i proteasomen.
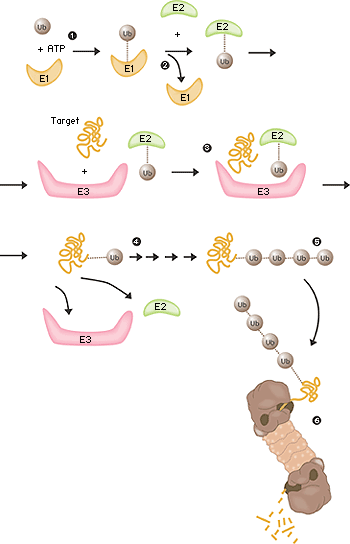
Fig 2. Ubiquitinmedierad nedbrytning
- Enzymet E1 aktiverar ubiquitin-molekylen. Denna reaktion kräver energi i form av ATP.
- Ubiquitin-molekylen förs över till ett annat enzym, E2.
- Enzymet E3 har förmågan att känna igen det protein som ska förstöras. Likaså fäster E2-ubiquitin-komplexet så nära proteinet att själva ubiquitin-etiketten kan föras över från E2 till proteinet.
- Enzymet E3 släpper nu taget om det ubiquitin-märkta proteinet.
- Detta sista steg upprepas tills proteinet har en kort kedja av ubiquitin-molekyler fäst till sig.
- Denna ubiquitin-kedja känns igen i proteasomens öppning. Ubiquitin-etiketten kopplas loss och proteinet släpps in och hackas i småbitar.
Alla studier fram till nu hade gjorts i cellfria system. För att även kunna studera den fysiologiska funktionen av ubiquitinmedierad proteinnedbrytning utarbetade Avram Hershko och hans medarbetare en immunkemisk metod. Genom att använda antikroppar mot ubiquitin kunde man isolera ubiquitin-protein-konjugat från celler där cellproteinerna hade pulsmärkts med en radioaktiv aminosyra som inte finns i ubiquitin. Resultaten visade att celler verkligen bryter ned felaktiga proteiner med hjälp av ubiquitin-systemet och vi vet nu att upp till 30% av nysyntetiserade proteiner i en cell bryts ner via proteasomen eftersom de inte klarar cellens rigorösa kvalitetskontroll.
Proteasomen – cellens avfallskvarn
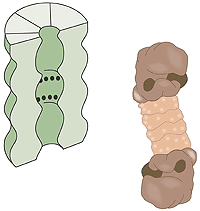
Vad är en proteasom? En mänsklig cell innehåller cirka 30 000 proteasomer. Dessa tunnformade strukturer kan bryta ner i stort sett alla proteiner till peptider som är 7–9 aminosyror långa. Den aktiva ytan i proteasomen sitter inne i tunnan där den är avskärmad från resten av cellen. Enda vägen in till aktiva ytan är genom “locket”, vilket känner igen polyubiquitinerade proteiner, denaturerar dem med hjälp av ATP energi och släpper in proteinet i tunnan för nedmontering sedan först ubiquitin-etiketten avlägsnats. De peptider som bildas släpps ut från proteasomens andra ända. Proteasomen själv kan alltså inte välja mellan olika proteiner utan det är främst E3-enzymet som genom att ubiquitin-etikettera rätt protein väljer ut det för nedbrytning (fig. 3).
 |
| Högupplöst bild (jpeg 187 kB) |
| Fig 3. Cellens”avfallskvarn”, proteasomen. De svarta punkterna indikerar aktiva, proteinnedbrytande ytor. |
Utan kvalitetskontroll blir det produktionsstopp
När de biokemiska mekanismerna bakom den ubiquitinmedierade proteinnedbrytningen var klarlagda omkring 1983 hade man ännu inte insett dess fulla fysiologiska betydelse. Att den har betydelse i att förstöra felaktiga intracellulära proteiner visste man, men för att komma vidare behövde man en cell som var muterad i ubiquitinsystemet. Genom att noga studera hur den muterade cellen skiljer sig från en normal cell under olika tillväxtbetingelser hoppades man kunna få en bättre uppfattning om vilka reaktioner i cellen som är beroende av ubiquitin-systemet.
En sådan muterad muscell isolerades 1980 av en forskargrupp i Tokyo. Deras muscell-mutant innehöll ett protein som på grund av mutationen var temperaturkänsligt. Vid lägre temperatur fungerade proteinet som det skulle men vid högre temperatur slutade det fungera. När cellerna odlades vid den högre temperaturen slutade de växa. Cellerna uppvisade dessutom defekt DNA-syntes och andra felaktiga funktioner vid den högre temperaturen. Forskare i Boston kunde snabbt visa att det värmekänsliga proteinet i muscell-mutanten var det ubiquitin-aktiverande enzymet E1. Uppenbarligen var ubiquitin-aktiveringen nödvändig för att cellen överhuvudtaget skulle fungera och föröka sig. Denna kontollerade proteinnedbrytning var alltså inte bara viktig för att bryta ner felaktiga proteiner i cellen utan deltog sannolikt även i kontrollen av cellcykeln, DNA-replikation och kromosomstruktur.
Från sent 1980-tal och framåt har en rad fysiologiskt viktiga substrat för ubiquitinmedierad proteinnedbrytning identifierats och här kommer endast några få av de viktigaste att nämnas.
Förhindrande av självpollinering hos växter
De flesta växter är tvåkönade, hermafroditer. Självpollinering leder till en gradvis minskning av den genetiska variabiliteten vilket på sikt kan medföra att hela arten dör ut. För att förhindra detta använder sig växter av ubiquitinmedierad proteinnedbrytning för att avstöta “själv”-pollen. Den exakta mekanismen är ännu inte klarlagd men E3-enzymet har påträffats och när man tillsatt proteasomhämmare har avstötningen försämrats.
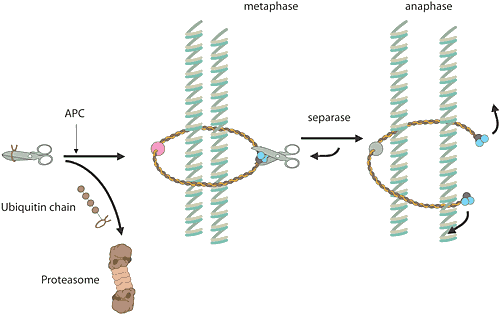
Reglering av cellcykelnNär en cell ska göra en kopia av sig själv, är det många kemiska reaktioner inblandade. För en människa rör det sig om sex miljarder baspar som ska dupliceras i DNA. Dessa ligger samlade i 23 kromosompar som ska kopieras. Den vanliga celldelningen, mitosen, liksom bildandet av könsceller, meiosen, har många beröringspunkter med årets Nobelpris. Det ansvariga E3-enzymet, ett proteinkomplex som kallas “anaphase-promoting complex” (APC), kontrollerar att cellen går ut ur mitosen. Enzymkomplexet har även visat sig ha en viktig roll vid separationen av kromosomerna under mitos och meios. Ett annat proteinkomplex fungerar som ett rep kring kromosomparen och håller samman dem. Vid en given signal ubiquitinmärker APC en hämmare av ett visst proteinnedbrytande enzym varpå hämmaren förs till proteasomen och blir förstört. Enzymet blir fritt, aktiveras och klipper upp repet kring kromosomparen. I och med att repet är borta kan kromosomparen separeras. Felfördelning av kromosomerna under meiosen är den vanligaste orsaken till spontana missfall under graviditeten och en extra kromosom 21 hos människa leder till Downs syndrom. De flesta elakartade tumörer har celler med förändrat kromosomantal som ett resultat av felfördelning av kromosomerna under mitosen. |
 |
| Högupplöst bild (jpeg 230 kB) |
DNA-reparation, cancer och programmerad celldöd
Proteinet p53 har kallats “genomets väktare” och det är ett s.k. tumörsupressorprotein. Det betyder att så länge en cell kan tillverka p53 försvåras uppkomsten av cancer. Proteinet är följdriktigt muterat i minst 50 % av alla humana cancer. Mängden p53-protein i en normal cell är låg till följd av en kontinuerlig tillverkning och nedbrytning. Nedbrytningen regleras genom ubiquitinering och det ansvariga E3-enzymet bildar ett komplex med p53-proteinet. Efter en DNA-skada fosforyleras p53-proteinet och det gör att det inte längre binder till sitt E3-enzym, nedbrytningen avstannar och mängden p53-protein i cellen höjs snabbt. p53-proteinet fungerar som en transkriptionsfaktor. En transkriptionsfaktor är ett protein som reglerar uttrycket av en viss gen. p53-proteinet binder till och kontrollerar gener som reglerar reparation av DNA och programmerad celldöd. Höjda nivåer av p53-protein leder först till stopp i cellcykeln för att ge tid till reparation av en DNA-skada. Om skadan är för omfattande utlöser cellen programmerad celldöd och “begår älvmord”.
Infektion med humant papillom-virus är starkt korrelerad till uppkomsten av livmoderhalscancer. Viruset undgår kontrollfunktionen hos p53-proteinet genom att ett virusprotein aktiverar och ändrar igenkänningsmönstret hos ett visst cellulärt E3-enzym, E6-AP, vilket luras att ubiquitinera p53-proteinet som helt förstörs. Till följd av detta kan den infekterade cellen inte längre reparera DNA-skador på ett normalt sätt eller utlösa programmerad celldöd, mutationerna i DNA blir allt fler och slutligen kan detta leda till cancerutveckling.
Immun- och inflammationsreaktioner
En viss transkriptionsfaktor reglerar många av de gener i cellen som är viktiga för immunförsvar och inflammationsreaktioner. Denna transkriptionsfaktor förekommer bundet till ett hämmarprotein i cellens cytoplasma och den bundna formen saknar aktivitet. När celler utsätts för bakterier eller en rad olika lokala signalsubstanser fosforyleras hämmarproteinet och det medför att det ubiquitineras och bryts ner i proteasomen. Den frisläppta transkriptionsfaktorn transporteras till cellkärnan där den binder till och aktiverar uttryck av specifika gener.
Ubiquitin-proteasomsystemet tillverkar även de peptider som presenteras av immunförsvaret på ytan av en virus-infekterad cell genom att bryta ned virusproteiner till lämplig storlek. T-lymfocyter känner igen dessa peptider och angriper cellen som ett viktigt led i vårt försvar mot virusinfektioner.
Cystisk fibros (CF)
Den ärftliga sjukdomen Cystisk fibros, CF, orsakas av en icke-fungerande plasmamembran-kloridkanal som kallas CFTR, “cystic fibrosis transmembrane conductance regulator”. De flesta CF-patienter har en och samma genetiska skada, en förlust av aminosyran fenylalanin i CFTR-proteinet. Mutationen förorsakar felveckning av proteinet och det i sin tur leder till att allt protein hålls kvar av cellens kontrollsystem för proteinkvalitet. Systemet ser till att det felveckade proteinet förstörs genom ubiquitinmedierad proteinnedbrytning i stället för att transporteras ut till cellväggen. En cell som saknar en fungerande kloridkanal kan inte längre transportera kloridjoner genom cellväggen. Detta påverkar sekretionen i bl.a. lunga och leder till ansamling av ett segt sekret i lungan vilket försämrar funktionen och starkt ökar risken för infektion.
Ubiquitinsystemet har blivit ett intressant forskningsområde för läkemedel mot olika sjukdomar. Sådana preparat kan riktas mot komponenter av det ubiquitinmedierade proteinnedbrytningssystemet för att hindra nedbrytningen av specifika proteiner. De kan även utformas så att de får systemet att förstöra oönskade proteiner. En medicin som redan testas kliniskt är proteasomhämmaren Velcade (PS341) som används mot multipelt myelom, en cancersjukdom som berör de antigen-producerande cellerna i kroppen.
Årets pristagare har förklarat den molekylära bakgrunden till ett för alla högre celler mycket viktigt regleringssystem för proteiner. Nya cellfunktioner som styrs av ubiquitinmedierad proteinnedbrytning upptäcks hela tiden och denna forskning pågår i ett stort antal laboratorier över hela världen.
| Pristagarna | |
| Aaron Ciechanover | |
| Technion (Israel Institute of Technology) Rappaport Institute 1 Efron Street P.O. Box 9697 Haifa 31096 Israel |
Israelisk medborgare, född 1947 (57 år) i Haifa, Israel. Doktorsgrad i medicin 1975 vid Hebrew University of Jerusalem, och i biologi 1982 vid Technion (Israel Institute of Technology), Haifa. Distinguished Professor vid Center for Cancer and Vascular Biology, the Rappaport Faculty of Medicine and Research Institute at the Technion, Haifa, Israel. |
| Avram Hershko | |
| Technion (Israel Institute of Technology) Rappaport Institute 1 Efron Street P.O. Box 9697 Haifa 31096 Israel |
Israelisk medborgare, född 1937 (67 år) i Karcag, Ungern. Doktorsgrad i medicin 1969 vid Hadassah and the Hebrew University Medical School, Jerusalem. Distinguished Professor vid avdelningen för biokemi, Rappaport Family Institute for Research in Medical Sciences at the Technion, Haifa, Israel. |
| Irwin Rose | |
| Dept. of Physiology and Biophysics College of Medicine University of California, Irvine Irvine, CA 92697 USA |
Amerikansk medborgare. Född 1926 (78 år) i New York, USA. Doktorsgrad 1952 vid University of Chicago, USA. Specialist vid avdelningen för fysiologi och biofysik på College of Medicine, University of California, Irvine, USA. |
Illustrationer: Typoform
Avram Hershko – Interview
Interview with the 2004 Nobel Laureates in Chemistry Aaron Ciechanover, Avram Hershko and Irwin Rose by Joanna Rose, science writer, 9 December 2004.
The Laureates talk about their respective background and education; how they met (8:44), their work together (12:33); their reactions when the discovery was made (16.41); and their present work (22:03).
Participating in the 2004 edition of Nobel Minds: the Nobel Laureates in Physics, David J. Gross and Frank Wilczek, the Nobel Laureates in Chemistry, Aaron Ciechanover, Avram Hershko and Irwin Rose, the Nobel Laureates in Physiology or Medicine, Richard Axel and Linda B. Buck and the Laureates in Economic Sciences, Finn E. Kydland and Edward C. Prescott. Program host is Nik Gowing.
Telephone interview with Professor Avram Hershko following the announcement of the 2004 Nobel Prize in Chemistry, 6 October 2004. The interviewer is science writer Joanna Rose.
Interview transcript
– Hello.
– Hello Avram. Congratulations to the prize. My name is Joanna Rose and I call from the Nobelprize.org, which is the official website of The Nobel Foundation.
– Yes.
– My congratulations to the prize.
– Thank you.
– How does it feel now?
– Oh, I am very happy. Very happy for my family, for my institution, my country, and for myself also. I think this is a very … as you know it is a very good recognition. I’m also very happy, I should add that … that Irwin Rose was included, because I got many prizes before, but he was never included. And he did make a very important contribution to the discovery. So I am glad that justice was made. I really think that justice was made at this time.
– Did you expect the message today?
– No. I was out on a picnic with four granddaughters. It is a holiday today in Israel. We call it a day of … a kids’ day. So I invited four grandchildren, and we went out for a picnic, and to a swimming pool in a kibbutz, and there I heard it from … somebody heard it on the radio.
– I understand.
– But it was good. It was very exciting.
– Yeah. What was your first reaction when you heard it from the radio?
– Well, I thought … I was very happy. My first reaction was I am very happy for Ernie Rose. And, also happy for myself, of course. And for Ciechanover.
– Can you tell me just how do you think that the Nobel Prize is going to affect your future work?
– I … you know I enjoy bench work very much. I try to do an experiment every day, even today. And, I would like to continue with that because it’s really exciting. So, I hope it won’t affect too much my life. But of course you never know. There will be distractions I am sure. And there will be some duties. I’m sure there will be some invitations I will have to say ”yes” to. But, more or less, I would like to continue to do my work. I think I can still contribute. Not in the same big way as twenty-five years ago, but still contributing and then still having a lot of fun at the bench.
– Did you realize, when you did your discovery for over twenty years ago, that it is worth a Nobel Prize?
– Yeah. I thought so. I wasn’t waiting for it you know, but I knew already that it … because the impact is really big, you know about … when I started to work on ubiquitin there were about ten papers a year on ubiquitin. And now there are thousands in a year. So, it really became a kind of a cascade, and many people heard about us all over the world … mind about us … very big about this … all over the world are working on different aspects of the ubiquitin system and different systems. So I knew it was important. But I wasn’t waiting for the prize. No, I wasn’t waiting for it. But of course, I am very grateful for it.
– I understand. Have you any good advice to young students that maybe dream about receiving the Nobel Prize in the future?
– Well, not about receiving the Nobel Prize, but about doing science. My advice is … well that’s what I did, you know, to try to find something novel, and open up new problems which is not yet reached a big level at this time, not yet interested, but you think is important. I think that’s what I did about thirty-five years ago. And then, continue with it. That’s my advice. Try to find a unique problem which is important, but which is not yet in the center of the attention of biology or of chemistry. I think that is true for discoveries, that’s how it should be done. So, that’s my advice for young people.
– Yeah. My last question is, have you ever visited the Nobel website?
– Pardon me?
– Have you ever visited the Nobel website on the internet?
– No.
– Um-hmm. So, now you will be there yourself.
– O.K.
– Yes, thank you very much and have a good day.
– Thank you. Same to you. Thanks for calling. Bye.
– Bye.
Did you find any typos in this text? We would appreciate your assistance in identifying any errors and to let us know. Thank you for taking the time to report the errors by sending us an e-mail.
Transcript from an interview with Avram Hershko

Avram Hershko during the interview.
Transcript from an interview with Avram Hershko, 2004 Nobel Laureate in Chemistry, at the 57th Meeting of Nobel Laureates in Lindau, Germany, July 2007. The interviewer is Adam Smith, Editor-in-Chief of Nobelprize.org.
Avram Hershko, it is a great pleasure to meet you.
Avram Hershko: Same here.
You were the co-recipient of the 2004 Nobel Prize in Chemistry for your part in the discovery of the ubiquitin-mediated protein degradation pathway. I would like to start by asking you some general questions.
Avram Hershko: Yes.
When I say the word mentorship, what does that conjure up?
Avram Hershko: Mentorship is important in science. You learn from reading, you learn from experience, you are creative but there is something that you have to learn from some other people. So it can be your supervisor for your PhD, it can be your colleagues, but it is important that you have some people to talk to and some people that you really appreciate. I personally was very fortunate with two mentors who kind of complemented each other. I had a very good biochemist as a supervisor for my PhD, after I did my MD I did a PhD, Jacob Mager who was an excellent biochemist and also a vigorous man., I learned from him to do controlled experiments which are very important.
 … in science you need both imagination and to control yourself …
… in science you need both imagination and to control yourself …And then I had another mentor in my post-doctoral fellowship, Gordon Tomkins, who had a very broad horizon, a great imagination. On the other hand he was not so strong in controls. So these two kind of complemented each other because in science you need both imagination and to control yourself. So I think I was very fortunate with my mentors.
A nice balance.
Avram Hershko: And then I was a mentor for Aaron Ciechanover but that is history.
So the two mentors you mentioned had very different profiles, Tomkins is very well known, Mager is less known.
Avram Hershko: He is less known. He is very well known in Israel but he was a scientist of the old kind in that he was interested in too many things. So he worked at the same time on about five or six different projects. And because of that he could not of course concentrate on one field and nowadays we have to. But as a student it was very useful for me because I had experience in many fields. So each project was in a completely different field so I got quite a broad education. But because of this he did not make an imprint in the scientific world, I do think he made an imprint through his students. I was his student and Aharon Razin who is very well known in DNA methylation was his student and a couple of others.
So he left a legacy for sure?
Avram Hershko: Left a legacy for definitely.
And as a student were you able to identify that he was too dissipated?
Avram Hershko: Yes, I identified it and I learned from it, so I was not dissipated. I did concentrate on one topic later on. But you know it is fun to be dissipated, it is not so useful.
I fear I know that to my own cost.
Avram Hershko: Yes.
When you choose students what do you look for?
Avram Hershko: I look for somebody who has a genuine interest in science, because that is the only reason to go into science. It is enthusiasm and the interest and being really enthusiastic about it. So I look for that, is he really interested in science? Of course I am trying to see whether he or she is intelligent. Some are very enthusiastic but in a kind of a non … They do not really understand what they are enthusiastic about. And the rest is I think is up to me, that is not having skills with their own hands and that I can teach them so I am not worried about that.
And how do you organise your lab environment? What is your perfect lab environment?
 … I think it is our duty to teach the graduate students …
… I think it is our duty to teach the graduate students …Avram Hershko: I like to have a small lab, I have always had a small lab. I have three to four graduate students and a technician and myself, it was always like that. And I purposefully kept it small because first of all I like to work myself, that is my hobby, bench work and I still do. So if I want to do bench work I can’t have a big group because then I would be busy with so many people. Also, the few students that I have, three or four students I want to give them sufficient attention. So because of that I always kept a small lab all my life and continue today. Also, I did not have too many post docs it was mostly graduate students. I think it is our duty to teach the graduate students.
That is very honourable but at the same time you have trained people in your way of thinking to become very useful around the lab and then you let them go.
Avram Hershko: Well, you have one example, Aaron Ciechanover who shared the Nobel Prize with me for his PhD. It is sufficient time.
It works.
Avram Hershko: It works.
And do you have any other physical principles by which you put the lab together which helps it work? Is there anything in particular?
Avram Hershko: Physical principles, I mean the basic equipment to be close by. Because Israel is a small country so we have to be careful about our equipment. So I teach each student how to use the equipment carefully in a considerate way so that others can use it and in a way that will preserve the equipment. So the lab is not a big lab but we have all the basic equipment and then we are quite careful about maintaining it. If that is what you meant.
That is one of the things I meant yes. Thank you. So let us turn a bit more to your history. You were born in 1937 in Hungary, and you and your family were caught up in the war and swept up in the deportations in the holocaust. It may be a foolish question but can you identify ways in which that shaped your future development as a scientist?
Avram Hershko: I am not sure that it shaped my career as a scientist. It definitely shaped my personality. The way I look at things, what is important, but I am not so sure about science. It was an experience, experience with the Nazis and over this period whether it shaped my career as a scientist … Actually I was interested in many things and I became interested in science only during my medical studies so it was a later event. I went to medicine and then during medical studies I got interested in basic sciences. But I am not sure that my personal experiences in the war shaped that. They do shape my outlook on the world and things like that of course.
What made you choose medicine as a subject?
Avram Hershko: It is a funny story and actually I wrote it up in my autobiography. I was interested in many things. I was interested in history, literature and when I finished high school it was a hard thing for me to decide what to choose. I have an older brother who went to medicine, who was a medical student and he said Why don’t you come to medicine, I already have books for you. So that is how I got to my medical sciences. But when I studied I got interested in the basic aspects of medicine. I finished medicine, I have an MD degree but I never practiced it much.
And you always planned to be a biochemist as soon as you …?
Avram Hershko: Yes, I took off one year, we did not have a formal MD-PhD programme at that time, but I took off one year during my medical studies and I went to a lab and then I knew that I was interested in biochemistry, that was actually already in the lab of Jacob Mager. So I finished medical studies, I finished internship. I think it is a good education, I think, to study medicine, if you want to be a biologist it is not a bad way to do it, study medicine, because you get a deep understanding of one organism, the human body. You learn much more than you can for example in biology, because you learn disciples such as pharmacology /- – -/ pathology, different diseases and then you see patients so you know what are the problems of bio-medical sciences today. I think it was a good way, a good biological education. That is how I look at it.
Interesting, so even if you are not going to practice medicine and you are never even going to apply yourself to clinical problems it is worth having that background?
Avram Hershko: I feel so, yes. I think so. At least for me it worked. Yes, this way, yes.
And bio-chemical approaches, classical bio-chemical approaches have characterised your life time’s work. Jumping ahead, was it a pleasure to you when you were awarded the Nobel Prize in Chemistry rather than in Physiology or Medicine? In a way of recognising that there is a link between chemistry and biology.
Avram Hershko: It was a pleasure. I realise that the Nobel Prize always regarded biochemistry as a part of chemistry, and so it is chemical processes in our body so even though the impact is in medicine, but the discovery was about chemical processes in our body, of protein cell labelled for destruction. As for using our classic bio-chemical techniques I always say that you have do in science what you have to do. So even though there was in the 1970’s, 1980’s a big revolution in technology in molecular biology but I saw that we would not be able to get there without physical biochemistry so that is why I continue to do biochemistry, that was the only way to find out how a completely new system works.
And it is still the case. There are still many genes without function.
 … in the beginning the only way to go was by biochemistry …
… in the beginning the only way to go was by biochemistry …Avram Hershko: Yes, yes. But now you have a very broad molecular genetic knowledge about the ubiquitin system. So it is very wise now to use also complementation, molecular genetic methods but of course you will need biochemistry in order to find out all the certain ubiquitin /- – -/ or it is regulated, so it is a combination now of biochemistry and molecular genetics. But in the beginning the only way to go was by biochemistry.
Do you still find sufficient numbers of people coming into biochemistry as a field?
Avram Hershko: Well it is more difficult than micro biology. You can not do everything with kits. But I think that people are beginning to realise there are limits to where you can go with genetics. Even with the most sophisticated techniques because we have the human genome but 60% of the human genome is genes of unknown function. And without biochemistry you will never know their function. So I think that people are realising that they have to use biochemistry, not only biochemistry, but in conjunction with other technologies to find out new functions of genes of unknown function.
Do all the new nomenclatures that are coined for subject surrounding if you like, the search for biological principles, systems, biology, functional genomics, these sorts of things? Do you think they confuse the picture a bit? Do you think they make it more difficult for students to know which way they should be heading?
Avram Hershko: Yes and no. To have systems in biology is a good idea, you know, you have to integrate things. I think it might be a bit too early yet you know, because you need to know more to put up with more depths. You can put up more depths when you have got a lot of knowns but when you have a lot of unknowns sometimes /- – -/ do not help you a lot. But in some cases they do. So I think system biology is now being fashionable because it is dangerous and it is good for some purposes and for some other purposes it is a slow /- – -/ which may actually hinder progress. That is my own thought about that.
If we turn to those extremely productive years of 1977 to 1981 when you were sorting out the basics of the ubiquitin pathway, there were really, it turns out, there were a team of three of you and what was it that, was there something special about that combination of personalities which led to the success of the project?
Avram Hershko: Yes, there was definitely a complementation. I was helped a lot by the collaboration with Ernie Rose. Ernie Rose was not in the field at all but I liked him so went to him for a sabbatical. His main work was enzyme mechanisms, that is what he was known for, how enzyme’s work. He has a very critical and sharp mind and I am kind of more intuitive, going by my nose. So I usually did an experiment which was kind of unexpected and he criticised me and that combination of intuitivness and criticism worked very well. And Aaron is a person of boundless energy, Aaron Ciechanover. He helped a lot with his huge energy and enthusiasm as a graduate student. So we all three are different people, three different generations, three different personalities but I think as a team we complemented each other very well.
It seems to have been a very wise choice to go to Ernie’s lab, particularly if he was not in the field, that is quite brave. It is a lovely idea that you go and work with a friend that who is outside the field, but it is novel.
Avram Hershko: Yes, but you know it was, I sometimes make such decisions.
It seems right.
Avram Hershko: I was in the Technion for seven years already and you know, we have a sabbatical every seventh year. And there was a small community of people who were interested in protein degradation and the field was quite awful at that time. Everybody had a pet theory without any basis or experimental evidence. So I did not feel like going to one of the people who were in the field of protein degradation. And I happened to meet Ernie Rose in a meeting a year before my sabbatical and we began to talk and I knew him, he was working on enzyme mechanisms, and I asked What else are you interested in? And he said that he was interested in protein degradation. I asked him How come you have never published anything in protein degradation? He said that “There is nothing worth publishing on protein degradation!”
He was interested because he was with a young investigator, he sat right next to Mel Simpson at Yale University, and Mel Simpson found, made the initial observation that when you add inhibitors of energy production to liver slices the degradation of protein stops so that integration was energy dependent. And then Mel Simpson went down to other things, protein synthesis and Ernie went down to enzyme mechanisms but he kind of, from time to time, he did some experiments on why energy is needed for protein degradation. He did not get anywhere so he did not publish. And he also saw that the field is not so good so that is why he say nothing is worth publishing on protein degradation. I like that. I like that he is a character and I thought to ask him Can I spend a sabbatical in your lab? and when I got there we already had the initial fractionations, fraction one, fraction two, and by the end of the sabbatical I asked him Can I bring my student over? and I brought over Aaron and that is when we made the breakthrough.
And was it counter intuitive finding that protein degradation required energy that had drawn you into the field? Was that what attracted you?
Avram Hershko: Yes, that is what attracted me … I would not think it is counter intuitive, it is counter what was known about the properties of protein, so it is giving you a clue that it is some kind a completely new mechanism. That I found with Gordon Tomkins, when I was a postdoc with Gordon Tomkins. And when I found it I actually found it earlier, similar observations were made by Mel Simpson so I was quite ignorant when I did my experiment. Energy inhibitors to liver cell cultures and found that the degradation of a certain enzyme tyrosine aminotransferase requires energy I was quite surprised because I thought it would be the opposite. But then I read up the literature and found that Simpson had found it.
Now Simpson saw that it means that protein synthesis is needed for protein degradation. But then I did an experiment, we had a good inhibitors of protein synthesis /- – -/ so then I saw that this was a completely new mechanism in which you need energy and it made sense because you need energy for selectivity and degradation of several proteins are highly selective. As opposed to the question of trypsin for example. So it was not counter intuitive it was a kind of a lead that gave me the thought that we had to look for a new system. And that is when I saw that I have to use biochemistry. So the original observation was actually made in culture cells.
And the selectivity of the system turns out to be much bigger than one ever would possibly have imagined. I assume that is the case? As the field has expanded and has come to take in lots of medical applications, have you moved with the field or have you stayed at the basic mechanism?
 … I do not know if I moved with the field but I moved ahead …
… I do not know if I moved with the field but I moved ahead …Avram Hershko: I do not know if I moved with the field but I moved ahead. Before that I went to work on the basic mechanism, but in the last 50 years I am working on the roles of the ubiquitin system and cell division in the cell division cycle. Because I was impressed by the finding of Tim Hunt of cyclins, a protein that is degraded at the end of mitosis. And he thought, he called it a cyclin protease but I saw that it must be the ubiquitin system so I began to work on that again by biochemistry. I used extracts from clam ocytes that were found by Joan Ruderman to facefully reproduce programmed cyclin degradation and so I found that ubiquitin mediated and there is a ubiquitin /- – -/ and shortly afterwards similar findings were made in the laboratory of Marquez in Harvard. So it was discovered at the same time. But I definitely went on from the basic mechanisms to the roles of the system in the cell, division cycle and that is what I am still interested in.
And was it the combination of the fact that it was Tim Hunt’s observation that brought you into the cell division field and your need for clam eggs that took you to Woods Hole on a regular basis?
Avram Hershko: Yes, correct. Because at first I tried to do it, I was on another sabbatical in Fox Chase and I tried to bring the clams to Fox Chase and then I found out it is much easier to bring myself to Woods Hole because you need a big supply.
And you go there every summer, is that correct?
Avram Hershko: Every summer, yes. I work there. It is a wonderful place to work.
What does it give you? Apart from the raw materials, what else does it provide?
Avram Hershko: It has a very great environment, scientific environment. Also in the summer lots of activities, excellent summer courses. Sometimes I go and hear lectures in the course of the students, good lectures. Very peaceful environment and for me, coming out from that pressure cooker of Israel, to get away, to a peaceful place for two months it gives you lots of stimulation in the way to think, to work. Also it is very convenient as I said I love bench work and I have there a small lab and I do experiments. It is all very close so we give the students quarters and my wife and I, there is dormitories there and the lab is 100 yards away. So I just, after dinner, go back and finish an experiment and it is all very convenient.
Do you work alone there?
Avram Hershko: I work alone with my own hands and I usually bring a graduate student because I think it is a good exposure for a graduate student to see what is going on there. As I said there is great activity of summer courses and good lecturers that are brought in during the summer. So both for work, together with me because it is a small lab, I rent a lab and you pay by the square feet so it has to be small. And when it is small it has advantages because I sit right next to the student so I tell him you do not hold the pipette in the right way and things like that.
Marvellous training for the student I guess.
Avram Hershko: Yeah usually it works well yes. I think I already brought four students in the past 12 years. They are used to it.
And then come the autumn it is back to Technion and I wanted to ask a little bit about that because it was quite a brave move to take the position at Technion originally. What prompted your decision to go there where you had to set up your own department in an engineering based university? What decided you to choose Technion in particular? Rather, for instance, I do not know, Hebrew University which already had a department.
Avram Hershko: Well it was, the story was a miscommunication. That is Mager, my mentor told me that he has this position for me at Hebrew University but he hated to write letters, at that time there were no e-mails, it was only letters. So post doctorate periods at that time were two years, not five years or more than that, like now. So after a year and a half I wrote him a letter, do I have a position? I got no answer. I wrote him another letter, no answer.
He was too busy.
Avram Hershko: I got his offer from the Technion because they started a new medical school so I said Ok. And then I got a big letter from Mager and it turned out that they had a position and he prepared the lab and everything so it was a case of a miscommunication, I suppose he just thought that it was clear to me that there is a position for me at the Hebrew University, which there was. They had a 10 year position and everything was prepared actually, the lab too.
 So I was kind of left with my own system and without input …
So I was kind of left with my own system and without input …But there were no phone calls at that time, only letters, and he hated to write letters. So that was to my … Looking back and I wrote it in my autobiography, I think it was good for me because Technion was a centre of technology but not of medical sciences. So at first kind of isolated new medical school, Technion, and sometimes isolation is good for you. ‘Splendid isolation’ as the British call it maybe. Because you do your own work, you are not bothered by anything else. So I was kind of left with my own system and without input or without any disturbance from others.
A little Woods Hole in Haifa.
Avram Hershko: Yes, in Haifa. Actually it was kind of like this Gregor Mendel … it was even a monastery, because until they built the medical school they put us in an old monastery that was used by the Vatican as a Christian school for girls. But then they ran out of students and so they rented it out, in this case to Technion. They never sell anything, they rent. So the whole ubiquitin system was discovered in that monastery.
That is a nice connection to the peace and quiet.
Avram Hershko: So you do not always need big buildings to do discoveries. And sometimes I think isolation is good for you when you work on a new field.
And you have been able to develop …
Avram Hershko: Because whenever I went to Jerusalem or to Weizmann they told me Why do you do old fashioned biochemistry? And I think, I still think that “old fashioned biochemistry” is what really allow the breaks.
And so now your department at Technion is the centre of old fashioned biochemistry.
Avram Hershko: Now my department and Technion medical school is one of the centres of bio-medical sciences in Israel. So you do a lot and also many /- – -/ people came in and still continue to do biochemistry because they know how to do that and not many other people know how to do that and they believe that it is needed. Right now I am working on the cell cycle and working on a certain aspect of a check point that is how certain ubiquitin ligase is regulated by the state of the mitotic spindle it is called the mitotic spindle assembly checkpoint. Which is very … It is a field in which many people work but most people do genetics or cell biology and I think that we have to learn, use again biochemistry to know how the system works, how the attachment …
It is the system that monitors the attachment of the chromosomes to the mitotic spindle and only when the last chromosome is attached to the spindle then these ubiquitin ligase that are loose exits from mitosis are deactivated, a ubiquitin inhibitor. And it is a very intricate system and dozens of proteins have been identified by these genetics and by /- – -/ in experiments but it is really not knowing how it is working. So it is like you have a play, you have a Hamlet but you do not know you have the bad king, the bad queen and you have Ophelia, but we do not even know what they are doing. So you know all the players but in order to find out how do they act you need biochemistry. So I am again applying biochemistry to a biological programme.
So this is really putting together the nuts and bolts of the error correction mechanism?
Avram Hershko: Yes. And for that I believe we need biochemists so I am doing that.
So one final question, the state of Israeli science and your hopes for how that will continue. Do you feel that things, there has been an incredible increase, exponential growth in Israeli science, is that set to carry on?
 I still think that the limiting factor in science is the brains and not the machinery and not huge grants …
I still think that the limiting factor in science is the brains and not the machinery and not huge grants …Avram Hershko: I hope so. Of course you need the resources and Israel is a small country. But I do believe that you can do good work with less resources, cannot do good work without any resources, but if you think well you can design experiments without wasting too much money and then you can do with smaller grants and small resources you can do with science. And I still think that the limiting factor in science is the brains and not the machinery and not huge grants. And they believe that Israel can make more contributions with its limited resources. Even though it is a small country and has many other problems.
I suppose the only potential problem on the horizon is the brains are being attracted away to the big jobs elsewhere.
Avram Hershko: There is always a brain drain, we have a good system, it is called a long fellowship. It is given by, it was made by some very wise people who viewed their faculty positions to post-doctoral fellows who come back to Israel. And the faculty position is given for three years so the university does not have to pay the salary for three years, or so they get some start up money provided that the university guarantees that it is a ten year tech position. So about ten or twelve are given out each year. And they are so prestigious that universities will find, even though they are hard up, they will find a position for that. And because of that the best can come back if they want you know. The best, the very best.
That is important.
Avram Hershko: And it is important, yes.
Yes. Ok, well thank you very much indeed for speaking to us.
Avram Hershko: Ok.
And I hope to see you again.
Avram Hershko: It was a pleasure.
Interview with the 2004 Nobel Laureate in Chemistry, Avram Hershko, at the 57th Meeting of Nobel Laureates in Lindau, Germany, July 2007. The interviewer is Adam Smith, Editor-in-Chief of Nobelprize.org.
Avram Hershko talks about the qualities he looks for in a student, the environment of his lab (4:56), and the importance of classic biochemical techniques in his research (10:54). He also reflects on how he came to work with Aaron Ciechanover and Irwin Rose (15:33), with whom he shared the 2004 Nobel Prize in Chemistry, the importance of his annual summer research trips to Massachusetts (23:47), and the challenges he faced in setting up a biochemistry unit at Technion in Israel (26:31).
Did you find any typos in this text? We would appreciate your assistance in identifying any errors and to let us know. Thank you for taking the time to report the errors by sending us an e-mail.
Irwin Rose – Nobel Lecture
Irwin Rose held his Nobel Lecture December 8, 2004, at Aula Magna, Stockholm University. He was presented by Professor Håkan Wennerström, Chairman of the Nobel Committee for Chemistry.
Irwin Rose held his Nobel Lecture December 8, 2004, at Aula Magna, Stockholm University. He was presented by Professor Håkan Wennerström, Chairman of the Nobel Committee for Chemistry.
Lecture Slides
Pdf 342 kB
Read the Nobel Lecture
Pdf 115 kB
Irwin Rose – Documentary
A short documentary about the lives and work of the 2004 Nobel Laureates in Chemistry Aaron Ciechanover, Avram Hershko and Irwin Rose, and their research on ubiquitin-mediated protein degradation.
Irwin Rose – Prize presentation
Watch a video clip of the 2004 Nobel Laureate in Chemistry, Irwin Rose, receiving his Nobel Prize medal and diploma during the Nobel Prize Award Ceremony at the Concert Hall in Stockholm, Sweden, on 10 December 2004.
Irwin Rose – Nobel diploma

Copyright © The Nobel Foundation 2004
Artist: Ingegerd Möller
Calligrapher: Annika Rücker
Avram Hershko – Banquet speech
Avram Hershko’s speech at the Nobel Banquet in the Stockholm City Hall, December 10, 2004.
Your Majesties, Your Royal Highnesses, Honored Laureates, Ladies and Gentlemen,
On behalf of the three of us, Irwin Rose, Aaron Ciechanover and myself, I would like to express our deep gratitude to the Nobel Foundation and to the Royal Swedish Academy of Sciences for bestowing upon us this greatest honor and scientific recognition. At times, a Nobel Prize is awarded to several people who did not work together, but contributed separately to a common discovery. At other times, the prize is given to a team of scientists whose collaborative research resulted in a discovery. However, it is rare that a Nobel Prize is bestowed upon a team of three, each of whom represents a different generation in science: a biochemist from Israel (myself), his graduate student at the time of the discovery (Aaron Ciechanover) and their host and collaborator in the United States (Irwin Rose). We each have a very different background, as well as very different personalities and talents. Perhaps, I may best describe myself as being intuitive and persistent, Ernie Rose as analytical and sharply critical and Aaron Ciechanover as a person of immense energies. It took the complementing talents and cumulative efforts of the three of us, together with huge efforts by dedicated research groups in Haifa and Philadelphia, building upon an important background of prior research, to reach the critical mass that resulted in the breakthrough in the research and the discovery of the ubiquitin system 25 years ago.
The discovery of this biochemical pathway has been recognized by awarding of the Nobel Prize in Chemistry, even though its implications are primarily in the biomedical sciences and hopefully, in the treatment or prevention of human diseases in the future. However, the boundaries between chemistry, biology, physics and medicine are rapidly disappearing. Only a comprehensive understanding of the chemical and physical processes in our cells and organ systems, will yield the insights needed to develop rational approaches to the prevention and treatment of disease. The Royal Swedish Academy of Sciences has recognized this intimate relationship between chemistry, biochemistry, physiology and medicine many times in the past, and now again with this year’s choice for the Nobel Prize in Chemistry.
At such an auspicious event, which recognizes the work of many years, one naturally feels the great debt owed for the support of the family and loved ones, without whom the achievement would not have been possible. Usually I thank only my wife and my own family, but now I am speaking for all three of us, and therefore I would like to thank all three spouses: Zelda Rose, Judy Hershko and Menuha Ciechanover. Thank you for many years of help and support, and for bearing with many times of absent mindedness, blank stares and strange moods when experiments didn’t go quite as expected. Thank you all.
Copyright © The Nobel Foundation 2004
Irwin Rose – Photo gallery
Irwin Rose receiving his Nobel Prize from
His Majesty the King Carl XVI Gustaf of Sweden at the Stockholm Concert Hall,
December 10, 2004.
Copyright © The Nobel Foundation 2004
American Irwin A. Rose acknowledges the applause after
receiving the Nobel Prize in Chemistry from the hands of His Majesty the King Carl XVI Gustaf of Sweden
Copyright © Pressens Bild AB 2004, S-112 88 Stockholm,
Sweden, Telephone: +46 (0)8 738 38 00
Photo: Sven Nackstrand
Photo: Hans Mehlin
Aaron Ciechanover – Nobel Lecture
Aaron Ciechanover held his Nobel Lecture December 8, 2004, at Aula Magna, Stockholm University. He was presented by Professor Håkan Wennerström, Chairman of the Nobel Committee for Chemistry.
Aaron Ciechanover held his Nobel Lecture December 8, 2004, at Aula Magna, Stockholm University. He was presented by Professor Håkan Wennerström, Chairman of the Nobel Committee for Chemistry.
Lecture Slides
Pdf 12.2 MB
Read the Nobel Lecture
Pdf 683 kB