Rudolph A. Marcus – Photo gallery
1 (of 6) Rudolph A. Marcus receiving his Nobel Prize from H.M. King Carl XVI Gustaf of Sweden at the Stockholm Concert Hall on 10 December 1992.
Nobel Foundation. Photo: Lars Åström
2 (of 6) Rudolph A. Marcus after receiving his Nobel Prize from H.M. King Carl XVI Gustaf of Sweden at the Stockholm Concert Hall on 10 December 1992.
Nobel Foundation. Photo: Lars Åström
3 (of 6) Laureates on stage at the Nobel Prize award ceremony at the Stockholm Concert Hall on 10 December 1992. From left: physics laureate Georges Charpak, chemistry laureate Rudolph A. Marcus, medicine laureates Edmond H. Fischer and Edwin G. Krebs.
Nobel Foundation. Photo: Lars Åström
4 (of 6) 1992 Nobel Prize laureates on stage at the Nobel Prize award ceremony at the Stockholm Concert Hall on 10 December 1992. From left: physics laureate Georges Charpak, chemistry laureate Rudolph A. Marcus, medicine laureates Edmond H. Fischer and Edwin G. Krebs, literature laureate Derek Walcott and laureate in economic sciences Gary S. Becker
Nobel Foundation. Photo: Lars Åström
5 (of 6) Rudolph A. Marcus delivering his speech of thanks at the Nobel Prize banquet in the Stockholm City Hall, Sweden, on 10 December 1992.
Nobel Foundation. Photo: Lars Åström
6 (of 6) Physics laureate Georges Charpak, chemistry laureate Rudolph A. Marcus and laureate in economic sciences Gary S. Becker photographed during Nobel Week in Stockholm, Sweden, December 1992.
Nobel Foundation. Photo: Lars Åström
The Nobel Prize in Chemistry 1992
Rudolph A. Marcus – Nobel Lecture
Nobel Lecture, December 8, 1992
Electron Transfer Reactions in Chemistry: Theory and Experiment
Read the Nobel Lecture
Pdf 980 kB
Rudolph A. Marcus – Banquet speech
Rudolph A. Marcus’ speech at the Nobel Banquet, December 10, 1992
Your Majesties, Your Royal Highnesses, Ladies and Gentlemen,
I deeply appreciate the great honor that Your Majesties and the Royal Swedish Academy of Sciences are bestowing on me today. I believe that it is the entire field of electron transfer, which reaches into many areas of chemistry and into biology, that is being recognized. Individuals in this room, and many others who are not here, have made tremendous contributions to this area of research. I did have the good fortune to learn about some important results at a relatively early stage in the development of this field, and to have the background to treat the problems. I’m not sure that I fully realized, until I saw the Academy’s fine poster on electron transfer, how many areas of practical life those processes enter into.
I think that the award recognizes another aspect which sometimes occurs in science as well as in other fields – simplicity and beauty. The lay person may not recognize, as I did not recognize in mathematics until I spent a year or more at a mathematical institute, that the beauty which a scientist can experience after deriving a simple equation or executing an incisive experiment is just as real as that which the artist may experience in creating a work of art.
I believe, too, that there are many analogies between the spoil of skiing, which I dearly love, and doing theoretical work in science – the challenge and sense of excitement when the slope is a little more difficult than one feels comfortable with, or the boredom if too easy, or the probable disaster if too difficult.
It is a pleasure to acknowledge my great debt to individuals in this room – Norman Sutin, John Miller, and Sven Larsson in the electron transfer field, Seymour Rabinovitch for his pioneering work in another area, unimolecular reactions, which has occupied almost half my time, and to my family – my wife Laura, whose positive outlook and companionship have been so important during our forty-three years of marriage, and our three sons, Alan, Kenneth, and Raymond, who have long outdistanced their father in skiing, and with whom we have shared so many happy experiences, I thank Your Majesties and the Academy for giving me the opportunity to share this great honor with you all.
Award ceremony speech
Presentation Speech by Professor Lennart Eberson of the Royal Swedish Academy of Sciences
Translation from the Swedish text
Your Majesties, Your Royal Highnesses, Ladies and Gentlemen,
The 1992 Nobel Prize in Chemistry is being awarded to Professor Rudolph Marcus for his contributions to the theory of electron transfer reactions in chemical systems. To understand the background of his achievements, we must transport ourselves back to the period around 1950, when chemistry looked completely different than it does today. In those days, it was still difficult to determine the structure of chemical compounds, and even more difficult to make theoretical calculations of the rate of chemical reactions.
Reaction rate is a fundamental concept in chemistry. A mixture of chemical compounds undergoes changes, or chemical reactions, at different rates. Today we can measure reaction rates using virtually any time scale from quadrillionths of a second to thousands of years. By the late 19th century, Sweden’s Svante Arrhenius;. later a Nobel Laureate, had shown that the rate of a chemical reaction can be described in terms of the requirement for a reacting system to cross an energy barrier. The size of this barrier was easy to determine experimentally. Calculating it was a formidable problem.
In the years after 1945, a new technique for determining reaction rates had been developed: the radioactive tracer technique. By substituting a radioactive isotope for a given atom in a molecule, new types of reactions could be studied. One such reaction was the transfer of an electron between metal ions in different states of oxidation, for example between a bivalent and a trivalent iron ion in an aqueous solution. This turned out to be a slow reaction, that is, it took place over a period of hours, something highly unexpected by the chemists of that day. Compared with an atomic nucleus, an electron is a very light particle. How could the slowness of its movement between iron ions be explained?
This problem led to lively discussion around 1950. Marcus became interested when he happened to read through some papers from a symposium on electron transfer reactions, where the American chemist Willard Libby had suggested that a well-known spectroscopic principle known as the Franck-Condon principle might apply to the movement of an electron between two molecules. Marcus realized that this ought to create an energy barrier, which might explain the slow electron transfer between bivalent and trivalent iron in an aqueous solution. To enable the two iron ions to exchange an electron, a number of water molecules in their surroundings must be rearranged. This increases the energy of the system temporarily, and at some point the electron can jump without violating the restrictions of the Franck-Condon principle.
In 1956, Marcus published a mathematical model for this type of reaction, based on classic theories of’ physical chemistry. He was able to calculate the size of the energy barrier, using simple quantities such as ionic radii and ionic charges. He later extended the theory to cover electron transfer between different kinds of molecules and derived simple mathematical expressions known as “the quadratic equation” and “the cross-equation.” These could be tested empirically and led to new experimental programs in all branches of chemistry. The Marcus theory greatly contributed to our understanding of such widely varying phenomena as the capture of light energy in green plants, electron transfer in biological systems, inorganic and organic oxidation and reduction processes and photochemical electron transfer.
The quadratic equation predicts that electron transfer reactions will occur more slowly the larger the driving force of the reaction is. This phenomenon received its own name, “the inverted region.” To a chemist, the phenomenon is just as unexpected as when a skier finds himself gliding more slowly down a slope the steeper it is. In 1965, Marcus himself suggested that certain chemiluminescent reactions (“cold light”) might serve as an example of the inverted region. Only after 1985, however, could further examples of such reactions be demonstrated. The most improbable prediction in his theory had thereby been verified.
Professor Marcus,
In the space of a few minutes, I have tried to trace and explain the origins of the theory of electron transfer that carries your name. Your theory is a unifying factor in chemistry, promoting understanding of electron transfer reactions of biochemical, photochemical, inorganic and organic nature and thereby contributing to science as a whole. It has led to the development of many new research programs, demonstrating the lasting impact of your work. In recognition of your contribution to chemistry, the Royal Swedish Academy of Sciences has decided to confer upon you this year’s Nobel Prize in Chemistry.
Professor Marcus, I have the honor and pleasure to extend to you the congratulations of the Royal Swedish Academy of Sciences and to ask you to receive your Prize from the hands of His Majesty the King.
Rudolph A. Marcus – Other resources
Links to other sites
Rudolph A. Marcus’s page at Caltech
Interview with Rudolph A. Marcus from The Vega Science Trust
‘Rudolph A. Marcus and His Theory of Electron Transfer Reactions’ from DOE R&D Accomplishments
Rudolph A. Marcus – Biographical

My first encounters with McGill University came when I was still in a baby carriage. My mother used to wheel me about the campus when we lived in that neighborhood and, as she recounted years later, she would tell me that I would go to McGill. There was some precedent for my going there, since two of my father’s brothers received their M.D.’s at McGill.
I have always loved going to school. Since neither of my parents had a higher education, my academic “idols” were these two paternal uncles and one of their uncles, my great-uncle, Henrik Steen (né Markus). My admiration for him, living in faraway Sweden, was not because of a teol.dr. (which he received from the University of Uppsala in 1915) nor because of the many books he wrote – I knew nothing of that – but rather because he was reputed to speak 13 languages. I learned decades later that the number was only 9! Growing up, mostly in Montreal, I was an only child of loving parents. I admired my father’s athletic prowess – he excelled in several sports – and my mother’s expressive singing and piano playing.
My interest in the sciences started with mathematics in the very beginning, and later with chemistry in early high school and the proverbial home chemistry set. My education at Baron Byng High School was excellent, with dedicated masters (boys and girls were separate). I spent the next years at McGill University, for both undergraduate and, as was the custom of the time, graduate study. Our graduate supervisor, Carl A. Winkler, specialized in rates of chemical reactions. He himself had received his Ph.D. as a student of Cyril Hinshelwood at Oxford. Hinshelwood was later the recipient of the Nobel Prize for his work on chemical kinetics. Winkler brought to his laboratory an enthusiastic joyousness in research and was much loved by his students.
During my McGill years, I took a number of math courses, more than other students in chemistry. Upon receiving a Ph.D. from McGill University in 1946, I joined the new post-doctoral program at the National Research Council of Canada in Ottawa. This program at NRC later became famous, but at the time it was still in its infancy and our titles were Junior Research Officers. The photochemistry group was headed by E.W.R. Steacie, an international figure in the study of free-radical reactions and a major force in the development of the basic research program at NRC. I benefitted from the quality of his research on gas phase reaction rates. Like my research on chemical reaction rates in solution at McGill (kinetics of nitration), it was experimental in nature. There were no theoretical chemists in Canada at the time, and as students I don’t think we ever considered how or where theories were conceived.
About 1948 a fellow post-doctoral at NRC, Walter Trost, and I formed a two-man seminar to study theoretical papers related to our experimental work. This adventure led me to explore the possibility of going on a second post-doctoral, but in theoretical work, which seemed like a radical step at the time. I had a tendency to break the glass vacuum apparatus, due to a still present impetuous haste, with time-consuming consequences. Nevertheless, the realization that breaking a pencil point would have far less disastrous consequences played little or no role, I believe, in this decision to explore theory!
I applied in 1948 to six well-known theoreticians in the U.S. for a postdoctoral research fellowship. The possibility that one of them might take on an untested applicant, an applicant hardly qualified for theoretical research, was probably too much to hope for. Oscar K. Rice at the University of North Carolina alone responded favorably, subject to the success of an application he would make to the Office of Naval Research for this purpose. It was, and in February 1949 I took the train south, heading for the University of North Carolina in Chapel Hill. I was impressed on arrival there by the red clay, the sandy walks, and the graciousness of the people.
After that, I never looked back. Being exposed to theory, stimulated by a basic love of concepts and mathematics, was a marvelous experience. During the first three months I read everything I could lay my hands on regarding reaction rate theory, including Marcelin’s classic 1915 theory which came within one small step of the Transition State Theory of 1935. I read numerous theoretical papers in German, a primary language for the “chemical dynamics” field in the 1920s and 1930s, attended my first formal course in quantum mechanics, given by Nathan Rosen in the Physics Department, and was guided by Oscar in a two-man weekly seminar in which I described a paper I had read and he pointed out assumptions in it that I had overlooked. My life as a working theorist began three months after this preliminary study and background reading, when Oscar gently nudged me toward working on a particular problem.
Fortunately for me, Oscar’s gamble paid off. Some three months later, I had formulated a particular case of what was later entitled by B. Seymour Rabinovitch, RRKM theory (“Rice-Ramsperger-Kassel-Marcus”). In it, I blended statistical ideas from the RRK theory of the 1920s with those of the transition state theory of the mid-1930s. The work was published in 1951. In 1952 I wrote the generalization of it for other reactions. In addition, six months after arrival in Chapel Hill, I was also blessed by marriage to Laura Hearne, an attractive graduate student in sociology at UNC. She is here with me at this ceremony. Our three sons, Alan, Kenneth and Raymond, and two daughters-in-law are also present today.
In 1951, I attempted to secure a faculty position. This effort met with little success (35 letters did not yield 35 no’s, since not everyone replied!). Very fortunately, that spring I met Dean Raymond Kirk of the Polytechnic Institute of Brooklyn at an American Chemical Society meeting in Cleveland, which I was attending primarily to seek a faculty position. This meeting with Dean Kirk, so vital for my subsequent career, was arranged by Seymour Yolles, a graduate student at UNC in a course I taught during Rice’s illness. Seymour had been a student at Brooklyn Poly and learned, upon accidentally encountering Dr. Kirk, that Kirk was seeking new faculty. After a subsequent interview at Brooklyn Poly, I was hired, and life as a fully independent researcher began.
I undertook an experimental research program on both gas phase and solution reaction rates, wrote the 1952 RRKM papers, and wondered what to do next in theoretical research. I felt at the time that it was pointless to continue with RRKM since few experimental data were available. Some of our experiments were intended to produce more.
After some minor pieces of theoretical study that I worked on, a student in my statistical mechanics class brought to my attention a problem in polyelectrolytes. Reading everything I could about electrostatics, I wrote two papers on that topic in 1954/55. This electrostatics background made me fully ready in 1955 to treat a problem I had just read about on electron transfers. I comment on this next period on electron transfer research in my Nobel Lecture. About 1960, it became clear that it was best for me to bring the experimental part of my research program to a close – there was too much to do on the theoretical aspects – and I began the process of winding down the experiments. I spent a year and a half during 1960-61 at the Courant Mathematical Institute at New York University, auditing many courses which were, in part, beyond me, but which were, nevertheless, highly instructive.
In 1964, I joined the faculty of the University of Illinois in Urbana-Champaign and I never undertook any further experiments there. At Illinois, my interests in electron transfer continued, together with interests in other aspects of reaction dynamics, including designing “natural collision coordinates”, learning about action-angle variables, introducing the latter into molecular collisions, reaction dynamics, and later into semiclassical theories of collisions and of bound states, and spending much of my free time in the astronomy library learning more about classical mechanics, celestial mechanics, quasiperiodic motion, and chaos. I spent the academic year of 1975-76 in Europe, first as Visiting Professor at the University of Oxford and later as a Humboldt Awardee at the Technical University of Munich, where I was first exposed to the problem of electron transfer in photosynthesis.
In 1978, I accepted an offer from the California Institute of Technology to come there as the Arthur Amos Noyes Professor of Chemistry. My semiclassical interlude of 1970-80 was intellectually a very stimulating one, but it involved for me less interaction with experiments than had my earlier work on unimolecular reaction rates or on electron transfers. Accordingly, prompted by the extensive experimental work of my colleagues at Caltech in these fields of unimolecular reactions, intramolecular dynamics and of electron transfer processes, as well as by the rapidly growing experimental work in both broad areas world-wide, I turned once again to those particular topics and to the many new types of studies that were being made. Their scope and challenge continues to grow to this day in both fields. Life would be indeed easier if the experimentalists would only pause for a little while!
There was a time when I had wondered about how much time and energy had been lost doing experiments during most of my stay at Brooklyn Poly- experiments on gas phase reactions, flash photolysis, isotopic exchange electron transfer, bipolar electrolytes, nitration, and photoelectrochemistry, among others-and during all of my stay at NRC and at McGill. In retrospect, I realized that this experimental background heavily flavored my attitude and interests in theoretical research. In the latter I drew, in most but not all cases, upon experimental findings or puzzles for theoretical problems to study. The growth of experiments in these fields has served as a continually rejuvenating influence. This interaction of experiment and theory, each stimulating the other, has been and continues to be one of the joys of my experience.
Honors received for the theoretical work include the Irving Langmair and the Peter Debye Awards of the American Chemical Society (1978, 1988), the Willard Gibbs, Theodore William Richards, and Pauling Medals, and the Remsen and Edgar Fahs Smith Awards, from various sections of the ACS, (1988, 1990, 1991, 1991, 1991), the Robinson and the Centenary Medals of the Faraday Division of the Royal Society of Chemistry (1982, 1988), Columbia University’s Chandler Medal (1983) and Ohio State’s William Lloyd Evans Award (1990), a Professorial Fellowship at University College, Oxford (1975 to 1976) and a Visiting Professorship in Theoretical Chemistry at Oxford during that period, the Wolf Prize in Chemistry (1985), the National Medal of Science (1989), the Hirschfelder Prize in Chemistry (1993), election to the National Academy of Sciences (1970), the American Academy of Arts and Sciences (1973), the American Philosophical Society (1990), honorary membership in the Royal Society of Chemistry (1991), and foreign membership in the Royal Society (London) (1987) and in the Royal Society of Canada (1993). Honorary degrees were conferred by the University of Chicago and by Goteborg, Polytechnic, McGill, and Queen’s Universities and by the University of New Brunswick (1983, 1986, 1987, 1988, 1993, 1993). A commemorative issue of the Journal of Physical Chemistry was published in 1986.
This autobiography/biography was written at the time of the award and later published in the book series Les Prix Nobel/ Nobel Lectures/The Nobel Prizes. The information is sometimes updated with an addendum submitted by the Laureate.
Rudolph A. Marcus – Interview
Interview transcript
I am pleased to be sitting here with Rudolph Marcus on the 26th of June, the year 2000, which is actually the first day of the Nobel Prize Tagungen in Lindau which is actually the 50th anniversary of these days here. Let me first ask you that you were actually born in Canada, but your parents were immigrants.
Rudolph A. Marcus: My parents actually were immigrants, yes, but I was born in Canada. My father emigrated from the United States and my mother emigrated from England and they happened to meet in Canada. Yes, I’m a child of immigrants.
Many Laureates actually are sons and daughters of immigrants. Did they encourage you to go into higher studies?
Rudolph A. Marcus: I think that was the sort of atmosphere in my family. Now, I’m not sure if you’d regard the following as encouragement, but when I was a baby in a carriage, we lived near McGill University and my mother used to wheel me around McGill University and tell me that I would be going to McGill University. I suppose that’s a form of encouragement.
Surely.
Rudolph A. Marcus: Now the fact that two of my father’s brothers at the time were in medical school or had just finished medical school at McGill probably was the factor that maybe she thought that maybe I would go to McGill. She never urged me to go into medicine or anything like that, but I think that was a factor. Then I think another factor probably was just the atmosphere of education, partly those two uncles, but partly actually a great-uncle, whom I never met, in Sweden.
Yes.
 … he could speak 13 languages. Of course that’s a kind of encouragement …
… he could speak 13 languages. Of course that’s a kind of encouragement …Rudolph A. Marcus: What I learned about him is that he had emigrated to Sweden from Lithuania, just before the turn of the century, and then later changed his religion and had got a doctorate in theology in Uppsala. What I’d learned about him was that he could speak 13 languages. Of course that’s a kind of encouragement, you know? Then many years later, when I visited Sweden, actually before the Prize, I met two of his daughters, visited Sweden and then asked her about him because he had died in 1945, asked about him and I was very disappointed. I learned that no, he could not speak 13 language, he could only speak nine languages but one thing I did learn more – I knew he’d written some books because I’d seen a copy of the Swedish Who’s Who book from 1939 and his name in the form of Henrik Steen was in the book and it mentioned a number of books that he’d written on theology. I mean, for a while he was a representative of a Christian group in Jerusalem actually but anyway, see, he had taught at Gymnasium at Ventersburg for many years.
So when we went back to Sweden in 1992, I’d asked the sort of guide from the foreign ministry whether she could perhaps learn what books he had written and it turned out, much to my surprise, rather than just the few that are mentioned in the Who’s Who, that he had written 40 books. So in a sense some people might regard that as making up for the four missing languages. So that’s sort of the background and, for example, one of my uncles who went in medicine was very good at mathematics and as someone in high school his sister, my aunt, had given me a book that he’d won, a mathematics prize, so I think there was some mathematical interest in the family in that sense, anyway.
You told me that mathematics was actually your favourite …
 … one of the theories that I developed was very much like putting together pieces of the jigsaw puzzle …
… one of the theories that I developed was very much like putting together pieces of the jigsaw puzzle …Rudolph A. Marcus: I enjoyed mathematics just so much. I like the logic of, say, Euclidian geometry. I mean, whatever we were doing I just, I mean, certainly that was my favourite thing. Before that, of course, you know, as you know only too well, science is sort of figuring out things, trying to puzzle things out and I think that some of the work that I’ve done is related to some things I did when I was a child and many other children do. For example, as children we had jigsaw puzzles and I used to enjoy putting jigsaw puzzles and in fact I think one of the theories that I developed was very much like putting together pieces of the jigsaw puzzle; and another thing that, as children, many of us like to do or maybe grade school, possibly early high school, was we had these construction sets, Erector sets where you build things, where you work with your hands and you build things and I think that both of those are perhaps a reflection of the kind of spirit that many of us have in science and, you know, in trying to construct, trying to build, in the case of the mathematics, trying to think through things.
When you say that, I recollect my own experience with Meccano which was one of those marvellous …
Rudolph A. Marcus: I had a Meccano set too, yes. That’s right.
Now it’s Lego, I believe, it is taking over from that.
Rudolph A. Marcus: Yes, yes. I’m trying to remember, I think, in Canada Meccano came mainly from Britain and Erector from US, but I don’t remember. But those certainly were favourite pastimes.
So having McGill University nearby, it meant that you eventually …
Rudolph A. Marcus: Meant that even though we had very little money, it meant that I could go to university and in fact they did charge some tuition, well in fact for high school at that time tuition was charged, but fortunately in high school I won scholarships so I didn’t have to pay and at McGill they wanted at that time, it was during the second World War, accelerate science students to go through and finish and do various war work and so they had some special bursaries that they gave and so the college education didn’t cost anything either.
But you were not majoring in mathematics.
 … I think that put me off the physics and probably it’s just as well …
… I think that put me off the physics and probably it’s just as well …Rudolph A. Marcus: No, no. Those at McGill at the time could major in mathematics and physics. I think that was the choice. There may have been pure mathematics there but I don’t think so. Although the courses were pure mathematics, it was sort of math/physics major. That was one alternative and the other alternative for me was chemistry. I mean, there was just sort of a choice there and, although I liked mathematics so much, I think the physics that I had in high school sort of turned me off. Later on, when I had electricity and magnetism in college it was great, I enjoyed that very much, but in high school I remember all these levers and pulleys and I mean, no doubt many students have found that very easy, but I found it very difficult to translate and I think that put me off the physics and probably it’s just as well.
I say probably just as well because I remember in some of the math classes, although I was a very good student, I know for example in the complex variables course, I wasn’t first in the class. The student who was first was Louis Nirenberg who later shared the first Crafoord Prize with Arnold. Well anyway, seeing Louis do these proofs, these mathematical proofs, you know, the imagination, and the rest of us were sort of, you know, mechanically going away and he was so magic that I’m sure it’s a very good move not to go into mathematics.
After McGill, what happened next?
Rudolph A. Marcus: After McGill, there was a chance of applying for a fellowship to go to France and of course that would give one a chance to see Europe. It’s true, I’d been to Europe before, because when I was six months old my mother took me to see her parents and her family but I didn’t remember much from that time. So this would’ve been a chance to see Europe but somehow I felt I was kind of frivolous, you see, and so when somebody came down from the National Research Council of Canada, which was just beginning a post-doctoral programme, the NRC, as you know, in Ottawa, just beginning the post-doctoral programme, came down to seek people to apply for fellowships.
It was before the fellowship programme so we were called junior research officers you see, but we were doing the same thing that later on fellows did and so that was such a natural thing, so I sort of just drifted. In fact, I think all along, up to my first few years there, it had really been just sort of flowing, drifting and not sort of thinking great things or what have you, just flowing naturally. I mean, for example, there was no question I was going to be in science earlier on, but there was a question was it going to be math and that terrible statics in physics? Or whether it was going to be chemistry, so it ended up being chemistry but of course eventually it was the kind of chemistry where I was able to draw on physics, and that played a big role, androns, a little mathematics.
But you mentioned to me also that theory in Canada was virtually non-existent.
Rudolph A. Marcus: Not only virtually non-existent but in reality non-existent. In theory, in Canada at the time, there wasn’t a single theorist in all of Canada. Now there are many theorists but at that time there were none and so when we had courses, even though there was some theory described in some of the courses, it was theory described by experimentalists who were not as sophisticated as experimentalists like you in theory. But experiments at that time were much less sophisticated and so the people did their best, probably did a very good job, but we really didn’t have that much of a background so it was natural for me, when I went up to Canada, to do experimental work as I’d done for my Batchelor’s degree but after two or so years, I became dissatisfied inside, because although I enjoyed sort of working with my hands, there was something missing. In particular, things that I’d learned in mathematics I could hardly apply and in fact really not apply and I became increasingly conscious that I was missing something.
 … I decided we would form a two man seminar in which, it was like the blind leading the blind …
… I decided we would form a two man seminar in which, it was like the blind leading the blind …You know, at that time, for example, when I was at McGill and I, say, took a course in complex variables and all the other students were math/physics majors, they had courses in potential theories. They would be applying their complex … I had nowhere to apply it, no, so I really felt something was missing inside and then a friend and I, he was also a post-doctoral working with Stacey, a friend and I decided we would form a two man seminar in which, it was like the blind leading the blind, I would describe a theoretical paper to him then the next time he would describe one to me, you know. We’d try to learn theory that way and then ultimately applied to reactions that we were actually measuring the rates of. So that was the first time that we had to read the literature for that, the theoretical literature, and that was the first time that I was really conscious of theory as being a living subject and I began to think, and this was the first initiative that I really showed, that maybe I could try for post-doctoral in theory and, you know, 5,5 years of experimental background, maybe I could try for a post-doctoral.
So I applied to half a dozen people, but of course most of the people weren’t interested. I mean, here’s somebody with no theoretical background, you know, unknown and what have you, and so not all of the six replied but some of them did and they said, well one of them suggestion I applied at the Institute for Advanced Studies. Well, who’s going to take me? And so on, and another, you know, well another one wondered if I could maybe help guide his experimental group when I was on sabbatical. That was obviously not good. But then one of them, Oscar Rice, said that he would apply for an Office of Naval Research grant and if he got the grant, then I could come down as a post-doctoral and this was in the early days, this was before National Science Foundation. The Office of Naval Research was a real pioneer in the US in support of basic research. He applied for the grant, he got the grant and I went down and working with him was just marvellous. I mean, as an individual he was gentle, he was thoughtful of everybody, of broader issues, and he was exceedingly smart. I mean, he really examined things in detail. He was imaginative, he was an early person in reaction dynamics in the 1930s and in fact around 1936 I guess there was a choice, who was going to get the tenure position at Harvard? Rice or Bright Wilson? Bright Wilson was in structure, he was in dynamics. Bright Wilson got the position, Rice then went on to Carolina, but he turned out to be a marvellous choice.
One of the things that, looking back, I think was just so wonderful is that for the first three months, essentially I knew no theory, for the first three months I just read, you know, read things but we had sort of a two man seminar and I would once a week, well I would see him more often than that, but once a week I would describe to him some paper that I had read, theoretical paper, and he would point out assumptions maybe that I’d overlooked, but it was that kind of almost selfless, you know, guidance and that continued for quite a while. After three months, he gently suggested that maybe it would be a good idea to work on some particular problem and then he suggested, you know, there is this field of unimolecular reactions and I had made a measurement of a unimolecular decomposition of a free radical and some other stuff, so I knew something about the experiment and so he made that suggestion. So that was fine and I read every paper that I could possibly relate to that theoretical paper. Some papers were in German. My German was very small but I probably learned as much German during the time I spent on the post doc as I did learn chemistry, you see.
Anyway, in fact I still have a word by word translation of one of Landau‘s famous papers. I have … try to understand so he suggested that, you know, I work on the contrary problem so I ended up with not being conscious of trying to develop a big theory or anything like that, but just trying to understand the sort of unimolecular decomposition better. The previous work that had been done in the field had been done many years earlier, in the 1920s, before quantum mechanics came into chemistry so what they did in the 1920s was wonderful but it was limited by knowing nothing about structures, what have you. So what I ended up doing was putting little bits and pieces together. You remember I mentioned jigsaw puzzles and that was it. I mean, as I took the ideas of the theory of the 1920s, the statistical ideas, then there are various things that had been done related to transition state theory. Some were right, some were wrong. The ones that were wrong or that I thought were wrong, I threw away. The right ones, I brought in.
 … I brought in these pieces and without realising it, I’d developed a theory …
… I brought in these pieces and without realising it, I’d developed a theory …So I brought in these pieces and without realising it, I’d developed a theory. I mean, it really didn’t come out as a great thing, a great plan, but I’d developed a theory and then a year or two later I refined it but his idea paid off because the first three months were study, the next three months was development of the theory, so that was it, and later that theory became widely used, known as RRKM theory but I mean, I thought it was interesting as an example of where something is developed sort of in a sense unexpectedly. You are trying to just build on some things that had been done before but then putting them together in a way that something a bit different comes out.
You mentioned to me also some time that you resent the attitude of some supervisors to regard students as a pair of hands rather than, say …
Rudolph A. Marcus: That’s right. One thing I remember so much about Oscar Rice was that for him, I think, the student and his development or her development was primary. Stacey, when I worked with him, didn’t regard students as a pair of hands. I mean, we worked on interesting projects. Winkler, who had a large number of students and with whom I had done my doctoral studies, didn’t regard students as a pair of hands. In fact, students here almost worshipped Winkler, you know, just wonderful interaction and probably that’s true in many cases nowadays but there’s such pressure, in some cases, to produce a lot of publications that the time to get a PhD is longer and sometimes I wonder whether in some cases the students are mainly a pair of hands. In some cases, they mechanically go through something, whether it’s using a computer programme or using an apparatus. Now I’m sure in many, many cases there’s a lot of thoughtfulness that goes in both on the part of the student and the supervisor but there certainly seem to be a number of these cases which are not really that, you know.
You eventually ended up at Caltech and this is quite a place, I would say, with quite a number of Nobel Prizes.
Rudolph A. Marcus: Right, right, because the history you’re getting there is a rather long way. I didn’t go straight to Caltech. I don’t know, you might be interested in a little of the history …
Absolutely, absolutely.
Rudolph A. Marcus: I mean, for example, when I was going to be finished up with the doctorate at Caltech … Well six months after I got down there I not only had developed RRKM theory, but I got married too. I met a charming North Carolina girl and we got married six months later, so really at some point after being down there for about two years, I had to, you know, try to find, I wanted to try to find a new job of some sort and I applied to quite a few universities. As I’ve often said, I applied to 35 universities. That was a lot at that time. I didn’t get 35 no’s because not everyone replied but none of them said yes and so then I looked into possible post-doctorates and there would’ve been two possibilities, not definite but I had begun to explore them.
One was with Fritz London, who was nearby at Duke University, but that would’ve been a little bit too much on the physics side. Another was with Robert Mulligan, that I began to explore, but I really didn’t want to go on another post-doctoral, I really wanted to start teaching. So I went with a friend to an American Chemical Society meeting and at that meeting they, you know, had a kind of a job fair and it turned out that somebody from the Polytechnic Institute of Brooklyn was looking for faculty members. Now Polytechnic Institute of Brooklyn was not one of the 35. I had not heard of it, I hadn’t applied to it. Anyway, he was looking, and it also turned out that during the time I was at Carolina, Oscar Rice had got ill and eventually it was the illness which maybe 20 years later led to his death, but he got ill and for some time, I taught his courses and I think it was one course a term that I was teaching and one of the students in that class had formerly been a student at the Polytechnic Institute of Brooklyn, had happened to meet Dean Kirk, who was looking for young faculty members. He mentioned to Kirk that I, you know, taught these classes and then an interview was arranged and Kirk asked me very few questions. The one I remember was do you like young people? Well I was young, I was sort of surprised. I said yes I like young people.
 … it turned out that this chemistry department was full of a bunch of eager beavers …
… it turned out that this chemistry department was full of a bunch of eager beavers …Anyway, they invited me up for an interview and then I was given the position and that’s where I got started and that’s where most of the electron transfer work was in fact done and it’s interesting that the Polytechnic at that time was not a well-known school. It had very little endowment but what it did have was young people who were extremely active and so the atmosphere was great. It was a polymer centre, Herman Mark actually was there so, I mean, he wasn’t that young but he was the centre, he was one of the catalysts. There were several other people who had come over from Europe who were well known who were there and it was extremely strong in electrical engineering, microwaves. There was a microwave research institute but it turned out that this chemistry department was full of a bunch of eager beavers, so the atmosphere was just, you know, exciting and even though some of us were not in polymers, the fact that so many very well known people came through, leading people in the field, because of the polymer institute, came through to give talks.
That sort of added to the excitement so for me it turned out that that was a great place to get started and in fact, the electron transfer work for which I eventually got the Prize, the electron transfer work got started in a sense because of some question that a student in one of my classes in statistical mechanics asked. He asked a question about the application of some of the ideas to polyelectrolytes, these long chain molecules with a lot of charges which were being actively studied there and in other places and so on, and he asked about whether some theory could be applied to them and so I did a lot of reading related to that and eventually wrote two papers on polyelectrolytes, then the course had read many books on electrostatics, so how do you formulate things? There are three different expressions in the literature for the free energy of the system, are these expressions different? Are they equivalent? So I did a lot of reading and showed they’re equivalent but showed other things. So when accidentally I came across this paper by Willard Libby, who later got the Nobel Prize for carbon dating, on electron transfer reactions and on an unusual explanation and having a back of the envelope electrostatic calculation for him.
I was so excited because it was clear that his basic idea of what’s involved in trying to explain some of the experimental data, the basic idea was right but there was something that felt strange about his follow up of the idea, something was missing, and this back of the envelope calculation was the thing that clued me in because of all this background that I’d had in electrostatics. Then I realised what was missing. I think it means as much today as it did then – violation of the law of conservation of energy. That’s right. And so the question was how could one bring in that striking thing that Libby had brought in but not violate the law of conservation of energy?
And I saw what happened, that before an electron could jump from one molecule to another, all of the molecules around the first thing had to be prepared for the jump and all of those had to be prepared for the jump there and then the electron could jump and so the problem became how do you calculate, you know, how they’re prepared? But the thing is, is that my entrance into the field of electron transfer was a series of, you might say, coincidences or accidents. I mean, having to read that paper, having to see, because of the electrostatic background that I’d acquired for a different reason, that there was something wrong and then putting two together, it was a kind of jigsaw puzzle again.
There is one special thing about today because today, the 26th of June, the year 2000, there’s been a joint announcement by groups in the United States and in Britain and elsewhere about the first published, I would say almost draft, not completed, but the human genome is now sort of put officially on paper, so that everyone can study it and read it and look at it. And there has been even a joint discussion between Bill Clinton and Tony Blair about this event and so I can say that this is a major event in the history of mankind, actually, not just science. Would you like to comment about this?
 … I think the future for young people is just immense …
… I think the future for young people is just immense …Rudolph A. Marcus: Yes, of course everything in that field is far from my own, but one thing has been clear over the years, is how much biology is drawing upon chemistry and physics so that in a sense biology has become so much more molecular and now with the human genome project and the business of understanding the genes and understanding disease on a molecular basis, you know, I think the future for young people is just immense. I mean, I know a little bit about certain narrow corners of biology and I’m excited by those corners, so imagine with a broad area.
One of the wonderful things, and of course the Nobel committee has recognised this, one of the wonderful things is how some of these complex structures had become known, determined and of course to understand some of these biological processes, or understand any chemical reaction, you have to know something about structure as a starting point. Now in chemistry, for simple molecules, that’s well understood. I mean, automatically we assume we know what the starting structures are and the final; we don’t know the intermediates, that we learn about but the starting we don’t. Well before this, in the biological area, as you know, not much was known in detail about structure but now so much is being known. Like, for example, the prize on photosynthesis and how that fitted into the detailed studies of reaction mechanisms involving photosynthesis, or the transfer of energy that’s picked up from the sunlight to this photosynthetic reaction centre, the structural information there, or very recently with the prize for the ATP synthase, how nature’s smallest rotary engine, I mean what a wonderful device, what an exciting device to have in one little sort of unit. An ion current going across, an electric field being applied, something rotating and in the process forming ATP, which is such an important agent. And, of course, these rotary motors and linear motors are sort of ubiquitous so I mean there’s surely plenty of excitement.
I know there are already quite a few experiments, of course, but I think that things are just opening up. What we have learned so far at the basic level for basic understandings has so many potentialities for applying to these more complex systems. You know, are we going to be able to use the same rules or is there going to be a kind of simplification, for example, of protein folding? Is somebody going to come out with something which says, ah, you don’t need everything at this complete level of detail, but this is the broad outline and if you understand that, you have understood a … I mean, I have no idea, it’s not my area, I don’t know, but I think there’s so many possibilities. Of course, that’s one area, ok? And certainly the genome is going to expand it into molecular medicine, which is already very active, but of course there are other frontiers where physics and chemistry and mathematics meet, you know, and construct all sorts of the nanostructures, molecular computers eventually, goodness knows what. I think it’s exciting for young people to have these opportunities, but of course there’s been excitement for a long time. Many of us were excited by it. Yes.
Thank you Professor Marcus, thank you very much.
Rudolph A. Marcus: Thank you.
Did you find any typos in this text? We would appreciate your assistance in identifying any errors and to let us know. Thank you for taking the time to report the errors by sending us an e-mail.
Press release

14 October 1992
The Royal Swedish Academy of Sciences has decided to award the 1992 Nobel Prize in Chemistry
to Professor Rudolph A. Marcus, California Institute of Technology, Pasadena, California, USA
for his contributions to the theory of electron transfer reactions in chemical systems
A theory close to reality
Professor Rudolph A. Marcus is being rewarded for his theoretical work on electron transfer – work which has greatly stimulated experimental developments in chemistry. The processes Marcus has studied, the transfer of electrons between molecules in solution, underlie a number of exceptionally important chemical phenomena, and the practical consequences of his theory extend over all areas of chemistry. The Marcus theory describes, and makes predictions concerning, such widely differing phenomena as the fixation of light energy by green plants, photochemical production of fuel, chemiluminescence (“cold light”), the conductivity of electrically conducting polymers, corrosion, the methodology of electrochemical synthesis and analysis, and more.
From 1956 to 1965 Professor Marcus developed his theory for what is perhaps the simplest chemical elementary process, the transfer of an electron between two molecules. No chemical bonds are broken in such a reaction, but changes take place in the molecular structure of the reacting molecules and their nearest neighbours. This molecular change enables the electrons to jump between the molecules.
Professor Marcus found simple mathematical expressions for how the energy of the molecular system is affected by these changes. With these he was able to calculate and explain the greatly varying rates measured for electron transfer reactions. In the mathematical connection the Marcus theory makes between theoretical and experimental quantities, experimental chemists gained a valuable tool. The theory has proved useful in the interpretation of many chemical phenomena, even though it was initially controversial at some points. Certain predictions turned out to conflict with what the chemists had expected, and were also difficult to confirm experimentally. We had to wait for the final experimental confirmation until the latter part of the 1980s.
Background
When two molecules in a solution exchange one or more electrons, there is a reduction/oxidation process (redox process) in which one molecule accepts the electrons (reduction) and the other loses them (oxidation). Several different mechanisms can underlie such reactions. The simplest is the transfer of one single electron from one molecule to another. Changes take place in the structure, both in the reacting molecules and in those of the solution medium. Because of all these changes the energy of the molecular system rises temporarily and enables the electron to jump between the molecules. Energy must thus be supplied for the electron to be able to cross an energy barrier. The size of the energy barrier determines the speed of the reaction. An electron transfer of this kind is the simplest chemical elementary process, and is eminently suitable for theoretical studies.
At the beginning of the 1950s it was possible to determine the speed of a number of electron transfers between inorganic ions. Some of the reactions turned out to be very slow, which was surprising in view of the fact that only one electron changed places. It was considered at the time that such an insignificant change should not give rise to any large energy barrier.
The prizewinner’s contributions
From 1956 to 1965 Marcus published a series of papers on electron transfer reactions. His work led to the solution of the problem of greatly varying reaction rates.
Marcus made two assumptions about the reacting molecules. First, they had to be very loosely bonded to each other during the course of the reaction for classical physical-chemical theory to apply. Secondly, he assumed that it is the solvent molecules in the immediate vicinity that change their positions, thus increasing the energy in the molecular system. The electron can only jump between two states that have the same energy, and this condition can be fulfilled only by increasing the energy for both molecules. Marcus found a simple mathematical formula for calculating this energy change and was thus also able to calculate the size of the energy barrier. Somewhat later he extended the theory to include the energy associated with changes in the bonds of the reacting molecules.
In addition, Marcus further developed his model by showing that energy barriers could be calculated as a sum of two terms characterising each of the two components of the reaction. Lastly, he derived a general connection between electron transfer speed and the free energy change of the reaction, its “driving force”.
The general equation is quadratic and describes a parabola (see figure). The formula has the interesting consequence, unexpected by the chemist’s intuition, that, for a sufficiently large driving force, the reaction ought to take place more slowly the larger the driving force becomes. This area even received a special name, “the inverted region”. In the 1960s this prediction ran completely counter to chemists’ expectations and, in addition, it was difficult to study reactions of this type experimentally. Marcus himself proposed in 1965 that chemiluminescence reactions of a certain type ought to represent the inverted region, but it was not until the end of the 1980s that other, more convincing, experimental verifications could be made.
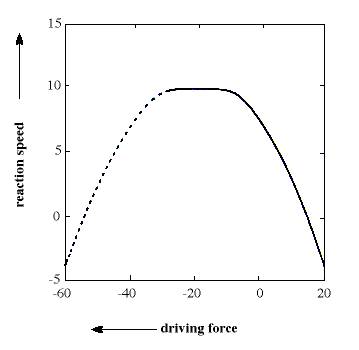
The figure shows a parabola which illustrates the connection between reaction speed (on a logarithmic scale) and driving force (expressed in kcal/mol) for an electron transfer reaction. In the left-hand part of the parabola (dashed line, the inverted region) reaction rate decreases with increasing driving force, a prediction that chemists long found difficult to accept and confirm.