Robert G. Edwards – Photo gallery
Mrs Ruth Edwards receiving Robert G. Edwards' Nobel Prize from His Majesty King Carl XVI Gustaf of Sweden at the Stockholm Concert Hall, 10 December 2010.
Copyright © The Nobel Foundation 2010
Photo: Frida Westholm
Mrs Ruth Edwards receiving Robert G. Edwards' Nobel Prize at the Stockholm Concert Hall, 10 December 2010.
Copyright © The Nobel Foundation 2010
Photo: AnnaLisa B. Andersson
Mrs Ruth Edwards and Professor Martin Hume Johnson showing the Nobel Medal and Diploma of Robert G. Edwards at the Stockholm Concert Hall, 10 December 2010.
Copyright © The Nobel Foundation 2010
Photo: AnnaLisa B. Andersson
Ruth Edwards (Robert G. Edward's wife) speaking at the Nobel Prize Symposium in Honour of Robert G. Edwards, 7 December 2010.
Copyright © The Nobel Foundation 2010
Photo: Orasisfoto
Robert G. Edward's wife, Ruth Edwards, and his daughters, at the Nobel Prize Symposium in Honour of Robert G. Edwards, 7 December 2010.
Copyright © The Nobel Foundation 2010
Photo: Orasisfoto
Professor Robert Edwards at the Bourn Hall 30th birthday celebrations for first "test tube baby" Louise Brown. Photo taken 12 July 2008.
Photo: Kindly provided by Bourn Hall Clinic.
Professor Robert Edwards, Lesley Brown, Louise Brown, the world's first "test tube baby" with her son Cameron. Photo taken 12 July 2008.
Photo: Kindly provided by Bourn Hall Clinic.
Louise Brown, the world's first "test tube baby" with her mother Lesley. Photo taken 9 October, 1978.
Photo: Brian Bould / Daily Mail / Rex Features /IBL Bildbyrå
Professor Robert Edwards at his desk at Bourn Hall Clinic, England. Photo taken in 1989.
Photo: CORBIN O'GRADY STUDIO/ Science Photo Library / IBL Bildbyrå
Human embryos developing in vitro. The photos show a fertilized egg, 8-cell stage, cell adhesion, a compacted morula, a blastocyst and zona hatching.
Photo: Wikipedia (http://en.wikipedia.org/wiki/File:Early_human_embryos.png)
A light micrograph image showing the encounter between sperm and ovum during in vivo fertilization. A single sperm is seen in centre of the image approaching the circular oocyte. Other competing sperm and cells encompass the corona radiata, which forms a protective halo around the central oocyte, are visible at bottom.
Photo: EDELMANN/SCIENCE PHOTO LIBRARY / IBL Bildbyrå
Robert G. Edwards – Other resources
Links to other sites
Obituary from The New York Times
Robert G. Edwards – Nobel Lecture
Nobel Lecture/Nobel Prize Symposium in Honour of Robert G. Edwards
The Nobel Prize Symposium in Honour of Robert G. Edwards was held on 7 December 2010 at Karolinska Institutet in Stockholm. The symposium was introduced by Professor Hugo Lagercrantz, member of the Nobel Assembly, and Mrs Ruth Edwards, wife of Robert G. Edwards.
The Nobel Prize Symposium in Honour of Robert G. Edwards was held on 7 December 2010 at Karolinska Institutet in Stockholm. The symposium was introduced by Professor Hugo Lagercrantz, member of the Nobel Assembly, and Mrs Ruth Edwards, wife of Robert G. Edwards.
Lecture Slides
Pdf 1.5 MB
Read the Lecture
Pdf 455 kB
Robert G. Edwards – Nobel diploma

Copyright © The Nobel Foundation 2010
Calligrapher: Susan Duvnäs
Book binder: Ingemar Dackéus
Photo reproduction: Lovisa Engblom
Robert G. Edwards – Prize presentation
Watch a video clip of Mrs Ruth Edwards receiving Robert G. Edwards’ Nobel Prize medal and diploma during the Nobel Prize Award Ceremony at the Concert Hall in Stockholm, Sweden, on 10 December 2010.
Illustrated information
Nobel Poster from the Nobel Committee for Physiology or Medicine, web adapted by
Nobelprize.org
Contents
The road to IVF
A historic delivery
Credits and references
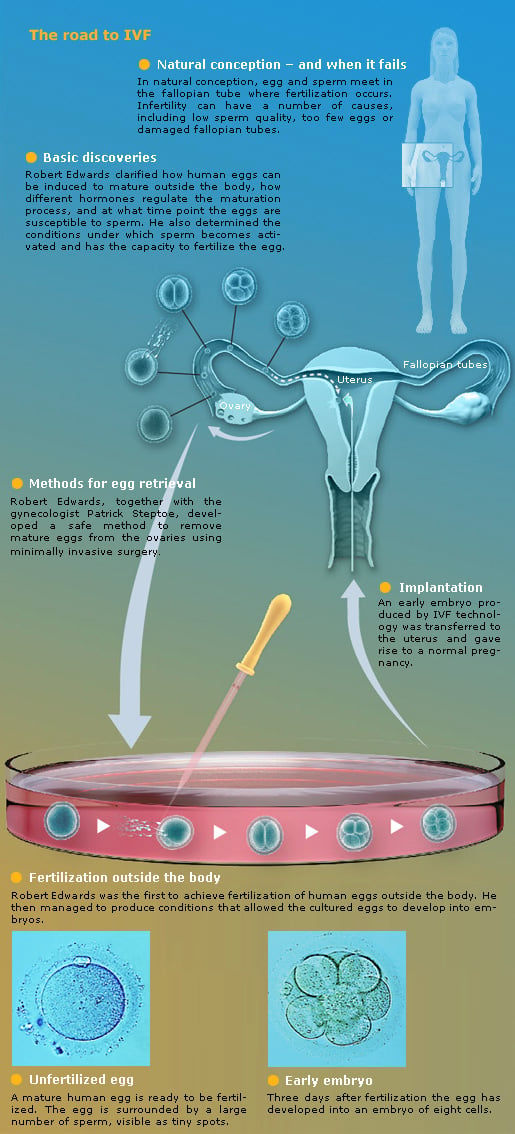
The Nobel Assembly at Karolinska Institutet has awarded the Nobel Prize in Physiology or Medicine 2010 to Robert G. Edwards for the development of human in vitro fertilization (IVF). His achievements have made it possible to treat infertility, a medical condition afflicting a large proportion of humanity including more than ten percent of all couples worldwide.

Robert G. Edwards
Robert Edwards was born in 1925 in Batley, Yorkshire, UK. During most of his academic career in reproductive physiology, he worked in Cambridge, UK, where he and his coworkers also started the world’s first IVF centre, Bourn Hall Clinic. Robert Edwards is currently professor emeritus at the University of Cambridge.
A historic delivery
On July 25th 1978 the world’s first IVF baby, Louise Brown, was born as a result of Robert Edwards’ new treatment. The event attracted worldwide attention and marked the beginning of a new era in medicine.
 |
IVF – a safe and effective treatment
IVF is now an established treatment when sperm and eggs cannot meet by natural means. Twenty to thirty percent of implanted eggs lead to the birth of a child. Complication risks are very small if only one egg is transferred into the uterus. Long-term follow-up studies have shown that IVF children are as healthy as other children.

Four million children – so far
Approximately four million children have so far been born with the help of IVF technology. Several IVF children have given birth to their own healthy children, and this is perhaps the best evidence for the safety and success of IVF therapy. Robert Edwards’ vision is now a reality, and brings joy to families all over the world
Credits and references for the 2010 Nobel Poster for Physiology or Medicine
Scientific Advisors, Professors at Karolinska Institutet: Göran K Hansson, Medicine, Secretary of the Nobel Assembly; Outi Hovatta, Obstetrics and Gynecology; Christer Höög, Genetics; Klas Kärre, Immunology, Chairman of the Nobel Committee; Hugo Lagercrantz, Pediatrics; Urban Lendahl, Genetics
Medical writer: Ola Danielsson
Illustrations and layout: Mattias Karlén
Copyright © 2010 The Nobel Committee for Physiology or Medicine
Web adapted version: Nobelprize.org
Nobel Prize® and the Nobel Prize® medal design mark are the registered trademarks of the Nobel Foundation.
The Nobel Prize in Physiology or Medicine 2010
Robert G. Edwards – Biographical
 For biographical information on Robert G. Edwards, see the lecture ‘Robert Edwards: Nobel Laureate in Physiology or Medicine’.
For biographical information on Robert G. Edwards, see the lecture ‘Robert Edwards: Nobel Laureate in Physiology or Medicine’.
Read the Lecture
Pdf 455 kB
Robert G. Edwards died on 10 April 2013.
Prize announcement
Announcement of the 2010 Nobel Prize in Physiology or Medicine to Robert G. Edwards, presented by Professor Göran K. Hansson, Secretary of the Nobel Committee for Physiology or Medicine, on 4 October 2010.
Detailed information about Robert G. Edwards’ work was presented by Professor Christer Höög.
Following the announcement, Professor Christer Höög told senior editor Simon Frantz how the 2010 Nobel Prize in Physiology or Medicine is unique in Nobel Prize history, as this year’s prize is the first awarded in the area of reproduction. Christer Höög explained that recent follow up studies showing that IVF children are as healthy as normally conceived children, were a contributing factor for awarding Robert G. Edwards the Nobel Prize in Physiology or Medicine in 2010 and not earlier.
Press release
English
English (pdf)
Swedish
Swedish (pdf)
![]()
2010-10-04
The Nobel Assembly at Karolinska Institutet
has today decided to award
The Nobel Prize in Physiology or Medicine 2010 to
Robert G. Edwards
for the development of in vitro fertilization
Summary
Robert Edwards is awarded the 2010 Nobel Prize for the development of human in vitro fertilization (IVF) therapy. His achievements have made it possible to treat infertility, a medical condition afflicting a large proportion of humanity including more than 10% of all couples worldwide.
As early as the 1950s, Edwards had the vision that IVF could be useful as a treatment for infertility. He worked systematically to realize his goal, discovered important principles for human fertilization, and succeeded in accomplishing fertilization of human egg cells in test tubes (or more precisely, cell culture dishes). His efforts were finally crowned by success on 25 July, 1978, when the world’s first “test tube baby” was born. During the following years, Edwards and his co-workers refined IVF technology and shared it with colleagues around the world.
Approximately four million individuals have so far been born following IVF. Many of them are now adult and some have already become parents. A new field of medicine has emerged, with Robert Edwards leading the process all the way from the fundamental discoveries to the current, successful IVF therapy. His contributions represent a milestone in the development of modern medicine.
Infertility – a medical and psychological problem
More than 10% of all couples worldwide are infertile. For many of them, this is a great disappointment and for some causes lifelong psychological trauma. Medicine has had limited opportunities to help these individuals in the past. Today, the situation is entirely different. In vitro fertilization (IVF) is an established therapy when sperm and egg cannot meet inside the body.
Basic research bears fruit
The British scientist Robert Edwards began his fundamental research on the biology of fertilization in the 1950s. He soon realized that fertilization outside the body could represent a possible treatment of infertility. Other scientists had shown that egg cells from rabbits could be fertilized in test tubes when sperm was added, giving rise to offspring. Edwards decided to investigate if similar methods could be used to fertilize human egg cells.
It turned out that human eggs have an entirely different life cycle than those of rabbits. In a series of experimental studies conducted together with several different co-workers, Edwards made a number of fundamental discoveries. He clarified how human eggs mature, how different hormones regulate their maturation, and at which time point the eggs are susceptible to the fertilizing sperm. He also determined the conditions under which sperm is activated and has the capacity to fertilize the egg. In 1969, his efforts met with success when, for the first time, a human egg was fertilized in a test tube.
In spite of this success, a major problem remained. The fertilized egg did not develop beyond a single cell division. Edwards suspected that eggs that had matured in the ovaries before they were removed for IVF would function better, and looked for possible ways to obtain such eggs in a safe way.
From experiment to clinical medicine
Edwards contacted the gynecologist Patrick Steptoe. He became the clinician who, together with Edwards, developed IVF from experiment to practical medicine. Steptoe was one of the pioneers in laparoscopy, a technique that was new and controversial at the time. It allows inspection of the ovaries through an optical instrument. Steptoe used the laparoscope to remove eggs from the ovaries and Edwards put the eggs in cell culture and added sperm. The fertilized egg cells now divided several times and formed early embryos, 8 cells in size (see figure).
These early studies were promising but the Medical Research Council decided not to fund a continuation of the project. However, a private donation allowed the work to continue. The research also became the topic of a lively ethical debate that was initiated by Edwards himself. Several religious leaders, ethicists, and scientists demanded that the project be stopped, while others gave it their support.
The birth of Louise Brown – an historic event
Edwards and Steptoe could continue their research thanks to the new donation. By analyzing the patients’ hormone levels, they could determine the best time point for fertilization and maximize the chances for success. In 1977, Lesley and John Brown came to the clinic after nine years of failed attempts to have a child. IVF treatment was carried out, and when the fertilized egg had developed into an embryo with 8 cells, it was returned to Mrs. Brown. A healthy baby, Louise Brown, was born through Caesarian section after a full-term pregnancy, on 25 July, 1978. IVF had moved from vision to reality and a new era in medicine had begun.
IVF is refined and spreads around the world
Edwards and Steptoe established the Bourn Hall Clinic in Cambridge, the world’s first centre for IVF therapy. Steptoe was its medical director until his death in 1988, and Edwards was its head of research until his retirement. Gynecologists and cell biologists from all around the world trained at Bourn Hall, where the methods of IVF were continuously refined. By 1986, 1,000 children had already been born following IVF at Bourn Hall, representing approximately half of all children born after IVF in the world at that time.
Today, IVF is an established therapy throughout the world. It has undergone several important improvements. For example, single sperm can be microinjected directly into the egg cell in the culture dish. This method has improved the treatment of male infertility by IVF. Furthermore, mature eggs suitable for IVF can be identified by ultrasound and removed with a fine syringe rather than through the laparoscope.
IVF is a safe and effective therapy. 20-30% of fertilized eggs lead to the birth of a child. Complications include premature births but are very rare, particularly when one egg only is inserted into the mother. Long-term follow-up studies have shown that IVF children are as healthy as other children.
Approximately four million individuals have been born thanks to IVF. Louise Brown and several other IVF children have given birth to children themselves; this is probably the best evidence for the safety and success of IVF therapy. Today, Robert Edwards’ vision is a reality and brings joy to infertile people all over the world.
Robert G. Edwards was born in 1925 in Batley, England. After military service in the Second World War, he studied biology at the University of Wales in Bangor and at Edinburgh University in Scotland, where he received his PhD in 1955 with a Thesis on embryonal development in mice. He became a staff scientist at the National Institute for Medical Research in London in 1958 and initiated his research on the human fertilization process. From 1963, Edwards worked in Cambridge, first at its university and later at Bourn Hall Clinic, the world’s first IVF centre, which he founded together with Patrick Steptoe. Edwards was its research director for many years and he was also the editor of several leading scientific journals in the area of fertilization. Robert Edwards is currently professor emeritus at the University of Cambridge.
References
Edwards RG. Maturation in vitro of human ovarian oocytes. Lancet 1965; 2:926-929.
Edwards RG, Bavister BD, Steptoe PC. Early stages of fertilization in vitro of human oocytes matured in vitro. Nature 1969; 221:632-635.
Edwards RG, Steptoe PC, Purdy JM. Fertilization and cleavage in vitro of human oocytes matured in vivo. Nature 1970; 227:1307-1309.
Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet 1978; 2:366.
Edwards RG. The bumpy road to human in vitro fertilization. Nature Med 2001; 7:1091-4.
 High resolution image (pdf 6 Mb)
High resolution image (pdf 6 Mb)
The Nobel Assembly, consisting of 50 professors at Karolinska Institutet, awards the Nobel Prize in Physiology or Medicine. Its Nobel Committee evaluates the nominations. Since 1901 the Nobel Prize has been awarded to scientists who have made the most important discoveries for the benefit of mankind.
Nobel Prize® is the registered trademark of the Nobel Foundation