Press release

NOBELFÖRSAMLINGEN KAROLINSKA INSTITUTET
THE NOBEL ASSEMBLY AT THE KAROLINSKA INSTITUTE
10 October 1994
The Nobel Assembly at the Karolinska Institute has today decided to award the Nobel Prize in Physiology or Medicine for 1994 jointly to
Alfred G. Gilman and Martin Rodbell
for their discovery of “G-proteins and the role of these proteins in signal transduction in cells”.
Summary
It has been known for some time that cells communicate with each other by means of hormones and other signal substances, which are released from glands, nerves and other tissues. It is only recently that we have begun to understand how the cell handles this information from the outside and converts it into relevant action – i.e. how signals are transduced in cells.
The discoveries of the G-proteins by the Americans Alfred G. Gilman and Martin Rodbell have been of paramount importance in this context, and have opened up a new and rapidly expanding area of knowledge.
G-proteins have been so named because they bind guanosine triphosphate (GTP). Gilman and Rodbell found that G-proteins act as signal transducers, which transmit and modulate signals in cells. G-proteins have the ability to activate different cellular amplifier systems. They receive multiple signals from the exterior, integrate them and thus control fundamental life processes in the cells.
Disturbances in the function of G-proteins – too much or too little of them, or genetically determined alterations in their composition – can lead to disease. The dramatic loss of salt and water in cholera is a direct consequence of the action of cholera toxin on G-proteins. Some hereditary endocrine disorders and tumours are other examples. Furthermore, some of the symptoms of common diseases such as diabetes or alcoholism may depend on altered transduction of signals through G-proteins.
Signal transduction in cells
We are made up of thousands of billions of cells that must act in concert to allow us to perform our daily activities and to meet challenges. This cooperation is achieved partly by cells communicating with each other through chemical signals. Hormones and other signal molecules are released from glands, nerves and other tissues. The chemical signals attach to specific recognition molecules, receptors, on the cell surface. These receptors transmit the signals to the interior of the cell. The important features of the communication between cells have been known for some time. On the other hand, the transduction of signals in cells was unclear until Alfred G. Gilman and Martin Rodbell made their discoveries.
The cell is surrounded by a membrane, largely composed of lipids, that effectively separates the outside of the cell from its inside. Earl Sutherland, USA, received the Nobel Prize in 1971 for his discoveries concerning the mechanism of action of hormones. He showed that the signal that is used to communicate between cells (“the first messenger”) is converted to a signal that acts inside the cell (“the second messenger”). It was known that this signal conversion occurred in the cell membrane, but not much more was understood about the processes involved.
Martin Rodbell and his coworkers at the National Institutes of Health in Bethesda, USA, demonstrated, in a set of pioneering experiments conducted in the late 1960’s and early 1970’s, that the signal transduction through the cell membrane involves a cooperative action of three different functional entities (Fig. 1).
It all starts with the chemical signal binding specifically to its receptor in the cell membrane. Since the receptor determines which signal molecules it will bind it functions, to use Rodbell’s nomenclature, as a discriminator.
The amplifier generates large amounts of the intracellular “second messenger”, for example cyclic AMP. Rodbell was one of the first to realize that the discriminator/receptor was distinct from the amplifier. However, his major discovery was the demonstration of a separate transducer function. It provides a link between the discriminator and the amplifier and thus plays a key role in signal transduction. Rodbell found that the transducer was driven by guanosine 5′-triphosphate, GTP, an energy rich compound. He also found that there may be several transducers.
 |
Figure 1. Martin Rodbell showed in 1971 that the transduction of a message from the exterior of the cell to its interior requires the cooperation of three functional units: 1) a discriminator (receptor) that recognizes different extracellular signals (first messengers), 2) a transducer that requires GTP, and 3) an amplifier that generates large quantities of a second messenger.
Alfred G. Gilman, working at the University of Virginia in Charlottesville, USA, decided to determine the chemical nature of Rodbell’s transducer. He used several kinds of leukemia cells with altered genetic setup. Gilman found that one mutated leukemia cell possessed a normal receptor and a normal amplifier protein that generated cyclic AMP as a second messenger. Despite this, the cell failed to respond normally when challenged with signals from outside – nothing happened.
Gilman showed that these mutated cells lacked the transducer function. After many years of work, he and his collaborators during the latter years of the 1970’s found – and in 1980 eventually purified – a protein in normal cells that when transferred into the membrane of the cell defective cell restored its function (Fig 2).
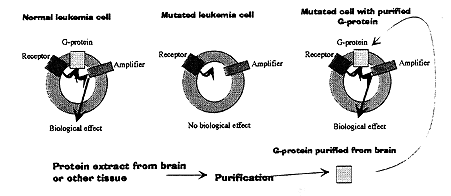
Figure 2. Alfred Gilman and his coworkers used leukemia cells to identify and demonstrate G-proteins. Normal leukemia cells respond with a normal biological response to the appropriate first messenger. In mutated cells, however, no response was evoked, because the cells lacked the G-protein. The function could be restored by G-protein derived from another tissue such as brain.
Thus, the first G-protein was discovered. It was given the name now commonly used, G-protein, because it reacts with GTP. Due to the discoveries of Gilman and Rodbell and their work, several laboratories turned to the area. Therefore we now know a great deal about the functions of G-proteins and how they control the activities of the cell.
A protein in shuttle service
G-proteins are composed of three separate peptide chains of different length, each existing in multiple forms. They are denoted alpha, beta and gamma, the first three letters of the Greek alphabet. All three are encoded by specific genes in the cell nucleus. Combinations of the different peptide chains allow the generation of some hundred different G-proteins. The alpha subunit, which is the largest, can bind GTP. When that happens, in a process stimulated by the receptor, the G-protein is converted to its active form. In this form it can turn on the formation of the second messenger, for example cyclic AMP. The G-protein converts GTP to GDP and reverts to an inactive form (Fig 3). The G-protein thus shuttles between the hormone receptor and the amplifier system in the cell membrane, being alternatively switched on or off.
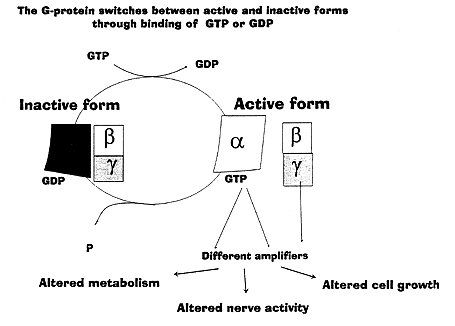 |
Figure 3. G-proteins act as molecular switches. The hormonal signal allows the G-protein to exchange GDP for GTP. The G-protein is thereby activated and several different amplifier systems can be activated. This leads to different changes in the cell. The signal is switched off again as GTP is converted to GDP.
There are thus several types of G-proteins. Each is activated by only some receptors and can in turn stimulate some specific amplifier systems. In this way characteristic responses in the cells are generated. In the retina of the eye there are specific G-proteins that convert the light signal to activation of those nerve fibers that convey visual stimuli to the brain. Our sense of smell depends on specific G-proteins in the olfactory cells, and the sensation of taste is related to yet other types of G-proteins.
Some G-proteins stimulate – other inhibit – the formation of cyclic AMP and hence the cellular metabolism. Some G-proteins alter the flux of ions over the cell membranes and thus the activity of the cell. G-proteins affect protein phosphorylation, and exert control over cell division and differentiation.
G-proteins and disease
Many symptoms of disease are explained by an altered function of G-proteins. A prime example is given by cholera, one of the most feared gastrointestinal infectious diseases. The disease is caused by cholera bacteria that produce a very poisonous cholera toxin. The toxin acts as an enzyme that alters one of the G-proteins in such a manner that it is locked in the active form. The traffic light is stuck on green. This prevents salt and water to be normally absorbed from the intestines. The resulting loss of water and salt can lead to dehydration and death. Symptoms after infection with some coli bacteria appear to have a similar background. A toxin produced by pertussis bacteria instead prevents the activation of some G-proteins. This can lead to a compromised immune defence.
In some common disease states the amounts of G-proteins in cells are altered. There can be too much or too little of them. In for example diabetes and in alcoholism there may be some symptoms that are due to altered signalling via G-proteins.
In animals it has been shown that a reduced expression of G-proteins can lead to altered development and to metabolic disturbances. In man it has been shown that mutated and overactive G-proteins are a characteristic of some tumors. An overactive G-protein is also found in a rare genetic endocrine disorder – McCune-Albrights syndrome – that is also characterized by so called cafe au lait spots on the skin. Yet another mutation of a G-protein, in this case causing a reduced activity, leads to disrupted calcium metabolism and skeletal deformations.
Nobel Prizes and laureates
See them all presented here.
