Popular information
Popular science background:
Electrons in pulses of light (pdf)
Populärvetenskaplig information:
Elektroner i blixtbelysning (pdf)

The Nobel Prize in Physics 2023
Through their experiments, this year’s laureates have created flashes of light that are short enough to take snapshots of electrons’ extremely rapid movements. Anne L’Huillier discovered a new effect from laser light’s interaction with atoms in a gas. Pierre Agostini and Ferenc Krausz demonstrated that this effect can be used to create shorter pulses of light than were previously possible.
Electrons in pulses of light
A tiny hummingbird can beat its wings 80 times per second. We are only able to perceive this as a whirring sound and blurred movement. For the human senses, rapid movements blur together, and extremely short events are impossible to observe. We need to use echnological tricks to capture or depict these very brief instants.
High-speed photography and strobe lighting make it possible to capture detailed images of fleeting phenomena. A highly focused photograph of a hummingbird in flight requires an exposure time that is much shorter than a single wingbeat. The faster the event, the faster the picture needs to be taken if it is to capture the instant.
The same principle applies to all the methods used to measure or depict rapid processes; any measurement must be done more quickly than the time it takes for the system being studied to undergo a noticeable change, otherwise the result is vague. This year’s laureates have conducted experiments that demonstrate a method for producing pulses of light that are brief enough to capture images of processes inside atoms and molecules.
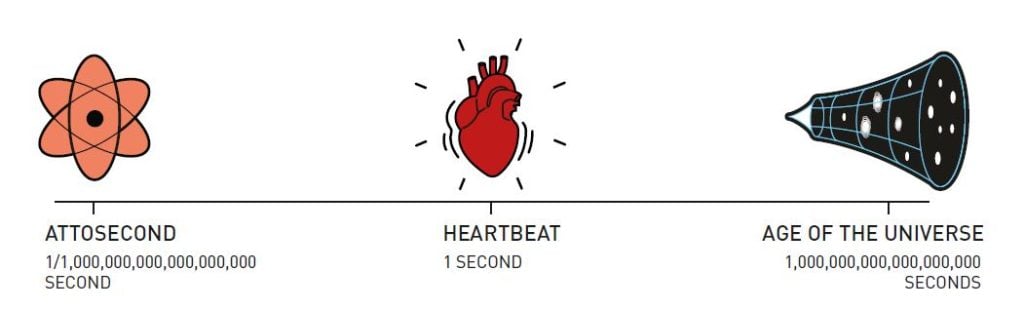
Atoms’ natural time scale is incredibly short. In a molecule, atoms can move and turn in millionths of a billionth of a second, femtoseconds. These movements can be studied with the very shortest pulses that can be produced with a laser – but when entire atoms move the timescale is determined by their large and heavy nuclei, which are extremely slow compared to light and nimble electrons. When electrons move inside atoms or molecules, they do it so quickly that changes are blurred out in a femtosecond. In the world of electrons, positions and energies change at speeds of between one and a few hundred attoseconds, where an attosecond is one billionth of a billionth of a second.
An attosecond is so short that that the number of them in one second is the same as the number of seconds that have elapsed since the universe came into existence, 13.8 billion years ago. On a more relatable scale, we can imagine a flash of light being sent from one end of a room to the opposite wall – this takes ten billion attoseconds.
A femtosecond was long regarded as the limit for the flashes of light it was possible to produce. Improving existing technology was not enough to see processes occurring on the amazingly brief timescales of electrons; something entirely new was required. This year’s laureates conducted experiments that opened up the new research field of attosecond physics.
Shorter pulses with the help of high overtones
Light consists of waves – vibrations in electrical and magnetic fields – that move through a vacuum faster than anything else. These have different wavelengths, equivalent to different colours. For example, red light has a wavelength of about 700 nanometres, one hundredth the width of a hair, and it cycles at about four hundred and thirty thousand billion times per second. We can think of the shortest possible pulse of light as the length of a single period in the light wave, the cycle where it swings up to a peak, down to a trough, and back to its starting point. In this case, the wavelengths used in ordinary laser systems are never able to get below a femtosecond, so in the 1980s this was regarded as a hard limit for the shortest possible bursts of light.
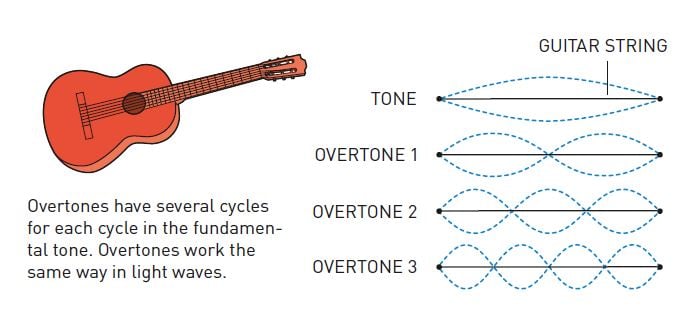
The mathematics that describes waves demonstrates that any wave form can be built if enough waves of the right sizes, wavelengths and amplitudes (distances between peaks and troughs) are used. The trick to attosecond pulses is that it is possible to make shorter pulses by combining more and shorter wavelengths.
Observing electrons’ movements on an atomic scale requires short-enough pulses of light, which means combining short waves of many different wavelengths.
To add new wavelengths to light, more than just a laser is necessary; the key to accessing the briefest instant ever studied is a phenomenon that arises when laser light passes through a gas. The light interacts with its atoms and causes overtones – waves that complete a number of entire cycles for each cycle in the original wave. We can compare this to the overtones that give a sound its particular character, allowing us to hear the difference between the same note played on a guitar and a piano.
In 1987, Anne L’Huillier and her colleagues at a French laboratory were able to produce and demonstrate overtones using an infrared laser beam that was transmitted through a noble gas. The infrared light caused more and stronger overtones than the laser with shorter wavelengths that had been used in previous experiments. In this experiment, many overtones of about the same light intensity were observed.
In a series of articles, L’Huillier continued to explore this effect during the 1990s, including at her new base, Lund University. Her results contributed to the theoretical understanding of this phenomenon, laying the foundation of the next experimental breakthrough.
Escaping electrons create overtones
When the laser light enters the gas and affects its atoms, it causes electromagnetic vibrations that distort the electric field holding the electrons around the atomic nucleus. The electrons can then escape from the atoms. However, the light’s electrical field vibrates continuously and, when it changes direction, a loose electron may rush back to its atom’s nucleus. During the electron’s excursion it collected lots of extra energy from the laser light’s electrical field and, to reattach to the nucleus, it must release its excess energy as a pulse of light. These light pulses from the electrons are what create the overtones that appear in the experiments.
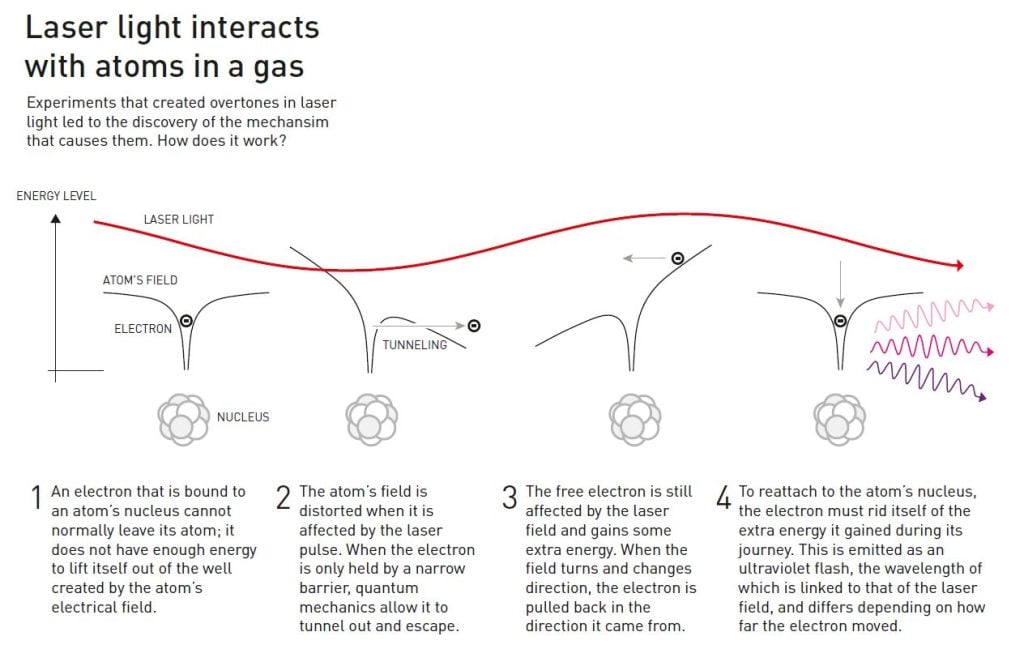
Light’s energy is associated with its wavelength. The energy in the emitted overtones is equivalent to ultraviolet light, which has shorter wavelengths than the light visible to the human eye. Because the energy comes from the laser light’s vibrations, the overtones’ vibration will be elegantly proportional to the wavelength of the original laser pulse. The result of the light’s interaction with many different
atoms is different light waves with a set of specific wavelengths.
Once these overtones exist, they interact with each other. The light becomes more intense when the lightwaves’ peaks coincide, but becomes less intense when the peak in one cycle coincides with the trough of another. In the right circumstances, the overtones coincide so that a series of pulses of ultraviolet light occur, where each pulse is a few hundred attoseconds long. Physicists understood the theory behind this in the 1990s, but the breakthrough in actually identifying and testing the pulses occurred in 2001.
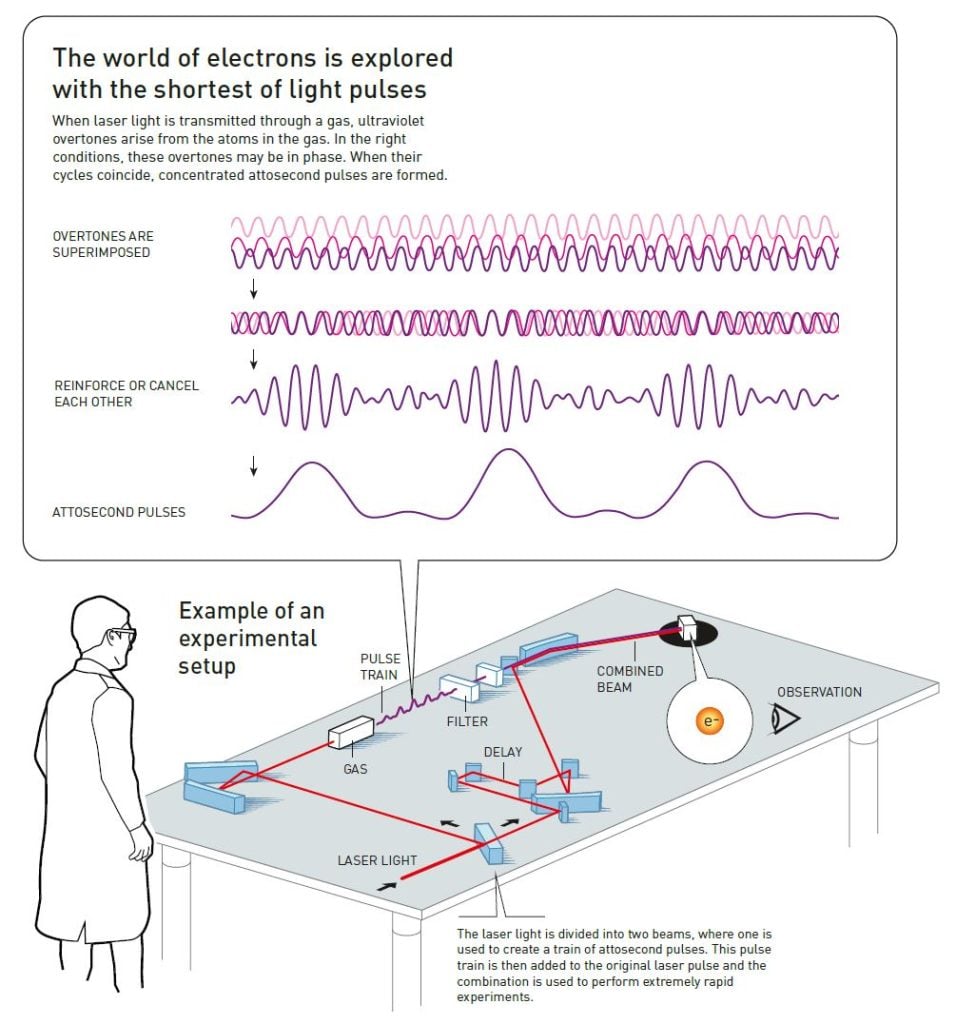
Pierre Agostini and his research group in France succeeded in producing and investigating a series of consecutive light pulses, like a train with carriages. They used a special trick, putting the “pulse train” together with a delayed part of the original laser pulse, to see how the overtones were in phase with each other. This procedure also gave them a measurement for the duration of the pulses in the train, and they could see that each pulse lasted just 250 attoseconds.
At the same time, Ferenc Krausz and his research group in Austria were working on a technique that could select a single pulse – like a carriage being uncoupled from a train and switched to another track. The pulse they succeeded in isolating lasted 650 attoseconds and the group used it to track and study a process in which electrons were pulled away from their atoms.
These experiments demonstrated that attosecond pulses could be observed and measured, and that they could also be used in new experiments.
Now that the attosecond world has become accessible, these short bursts of light can be used to study the movements of electrons. It is now possible to produce pulses down to just a few dozen attoseconds, and this technology is developing all the time.
Electrons’ movements have become accessible
Attosecond pulses make it possible to measure the time it takes for an electron to be tugged away from an atom, and to examine how the time this takes depends on how tightly the electron is bound to the atom’s nucleus. It is possible to reconstruct how the distribution of electrons oscillates from side to side or place to place in molecules and materials; previously their position could only be measured as an average.
Attosecond pulses can be used to test the internal processes of matter, and to identify different events. These pulses have been used to explore the detailed physics of atoms and molecules, and they have potential applications in areas from electronics to medicine.
For example, attosecond pulses can be used to push molecules, which emit a measurable signal. The signal from the molecules has a special structure, a type of fingerprint that reveals what molecule it is, and the possible applications of this include medical diagnostics.
Further reading
Additional information on this year’s prizes, including a scientific background in English, is available on the website of the Royal Swedish Academy of Sciences, www.kva.se, and at www.nobelprize.org, where you can watch video from the press conferences, the Nobel Lectures and more. Information on exhibitions and activities related to the Nobel Prizes and the Prize in Economic Sciences is available at www.nobelprizemuseum.se.
The Royal Swedish Academy of Sciences has decided to award the Nobel Prize in Physics 2023 to
PIERRE AGOSTINI
Born 1941 in Tunis, Tunisia. PhD 1968 from Aix-Marseille University, France.
Professor at The Ohio State University, Columbus, USA.
FERENC KRAUSZ
Born 1962 in Mór, Hungary. PhD 1991 from Vienna University of Technology, Austria.
Director at Max Planck Institute of Quantum Optics, Garching and
Professor at Ludwig-Maximilians-Universität München, Germany.
ANNE L’HUILLIER
Born 1958 in Paris, France. PhD 1986 from University Pierre and Marie Curie, Paris, France.
Professor at Lund University, Sweden.
“for experimental methods that generate attosecond pulses of light for the study of electron dynamics in matter”
Science Editors: Ulf Danielsson, Mats Larsson, Eva Olsson, the Nobel Committee for Physics
Text: Anna Davour
Translator: Clare Barnes
Illustrations: Johan Jarnestad
Editor: Sara Gustavsson
© The Royal Swedish Academy of Sciences
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 12 laureates' work and discoveries range from proteins' structures and machine learning to fighting for a world free of nuclear weapons.
See them all presented here.
