Roald Hoffmann – Banquet speech
Roald Hoffmann’s speech at the Nobel Banquet, December 10, 1981
Your Majesties, Your Royal Highnesses, Fellow Laureates, Ladies and Gentlemen,
Students of Stockholm, Students of all Sweden! I salute you for all that you represent:
For your youth, your beauty and your strength – symbolic of mankind’s constant renewal!
For your devotion to knowledge, your search for truth – typifying that most beautiful attribute of man- his mind!
For your country, its banners and its songs. A country dear to me through my wife and her family, through the Swedish people’s love for their land, their sea, and peace in the world!
For these things I, a child of war-torn Europe, of Jewish heritage and the American dream, salute you!
Students, the laureates share your sincere wish for our common goal as students of science, of literature, for peace in this world. Let it be in my time. Let it be in your time. Our energies and our hearts are devoted to it.
I do wish to add some words on the connections and differences among our respective fields. Each branch of scholarship honored by the Nobel Prize – physics, chemistry, physiology or medicine, literature, economics – gives birth to its own complexity, its own criteria of elegance, of understanding.
Chemistry, my own science, reduced to its simplest terms, is not physics. Medicine is not chemistry. And literature is certainly not economics. Or at least if so reduced each individual discipline loses much of its aesthetic, life-enhancing quality. Even if we could understand the sequence of firing of neurons in Pär Lagerkvist‘s mind when he wrote “Vem gick förbi min barndoms fönster och andades på det…” (“who walked past the window of my childhood and breathed on it…”); while beautiful in itself, knowledge of the specific physiological and eventually molecular sequence of events does not help us understand what this great Swedish poet has to say to us.
Students of Stockholm, each branch of learning we represent is unique and worthwhile in its own right. For the future of humanity I ask you to give each your strength and your love.
And to end I quote a poem about Van Gogh by Charles Tomlinson*:
“Farewell, and for your instructive frenzy
Gratitude. The world does not end tonight
And the fruit that we shall pick tomorrow
Await us, weighing the unstripped bough.”
*”Farewell to Van Gogh”, from Seeing is Believing (1958, 1960) in Collected Poems, Oxford University Press, 1985
Roald Hoffmann – Nobel Lecture
Nobel lecture, 8 December 1981
Building Bridges between Inorganic and Organic Chemistry
Read the Nobel Lecture
Pdf 308 kB
Roald Hoffmann – Interview
Interview transcript
What is science and what is science in relation to other ways for humans to deal with the wonders of life, like literature and poetry or music or arts or religion. This is what we are going to talk about today. Welcome to this interview Roald Hoffman. Maybe we will start with the first question. What is science?
Roald Hoffman: First I think science is a social system of western European invention, not an American one, for gaining reliable knowledge. I would say not truth, and we can talk about why there is a difference. And it has several components to it. It has curious people, some of whom are interested in mathematics or good at it. It has people who are willing to get their hands dirty, experimenters, not just philosophers. It has a system for communicating that knowledge and in fact a compulsion if not an addiction for doing so, exchanging that knowledge. And it is a system which makes use of pretty normal people to get interesting things to learn about the world around us and for us.
It has another aspect and which sometimes has been lost, it was over romanticised in the 19th century and that is that it should improve the human condition in some way. And that is implicit in Nobel’s will, it is interesting that it is there because it is a 19th century document. I would say like Peter Medawar does that at least we should ameliorate the human condition, to make it a little bit better. It is a little bit lost when science has become so professional as it is but I think it is important to revive it.
You also wrote something else about science in your book, with a colleague, ‘Old Wine, New Flasks’. You wrote ‘the purpose of science is to revive and cultivate a perpetual state of wonder’.
Roald Hoffman: I think science has a spiritual side to it. And that sense of wonder is what I refer to and that also sometimes, in the everyday struggle to gain knowledge, is lost, but it is part of what keeps people going. And knowing what the structure of DNA is, how this change and replicate in a body is just so wonderful. So there is a sense of wonder. In that sense I think the divisions that somehow science is materialistic and that there are other things which fulfil us spiritually, whether it is art, music or religion for some or literature, I think it is a little bit of a false division. I think there is spiritual value in science which sometimes scientists have lost themselves.
Now it is one way of knowing the world to get back to your initial question about other ways, it’s not the only way of knowing the world. And one of the things that is not so good about the science is given in its beginnings in an analytical tradition of the cart of dividing things up and then trying to compose them and then with a reductionist philosophy added to it that somehow understanding is defined by going deeper and deeper. What you have got is a set up of science that it has created a set of problems for itself which are capable of solutions. And that’s very different from what the arts and literature gives us and it deals with such a small part of the world. There are scientists who would say that other things too, like religion and art eventually, come out from some genetic predispositions and manipulations of our culture.
I’m thinking of Wilson’s socio-biology and things like that. But I think there are different ways of understanding this world. There is a reductionist way. Let’s see. If someone sends me a poem, an anonymous poem, and it is a line from John Dunne: ‘Love is a growing or full constant light and his first moment afternoon is night’. Knowing the sequence of firing those neurons in John Dunn’s mind when he wrote that poem or in a person with a mind, or the person who sent me that poem, or in my mind as I read it, knowing the sequence of beautiful biochemical actions and behind the firing of the neurons, that will get you a lot of Nobel Prizes but it has nothing in the world to do with understanding that poem.
That poem is understood on the level of the language and the psychology of the moment in terms of questions that seem, to some scientists, questions that are circular reasoning. And because you are using the same words to define the question as you are trying to explain them and yet that is meaning that is to be found. I think scientists sometimes get a little bit arrogant. And thinking about all they can do with the powers that they have and then they get upset when people outside of science still value religion or art, I do not think scientists are jealous of art but … religion are a little strange. So I think science is not …
Are there any similarities between the scientific way of searching for answers and for example religious or for artistic?
Roald Hoffman: Well, let’s talk about religion and science a little bit. I think religion and science come out initially from some of the same roots and that is a desire to understand the world. The mysteries of the world. The lightning, the lights, the stars, the fine patterns in it. Now they start out also for many religions with a feeling that the world is real. Some religions have added on that the real world does not matter and what matters is the afterlife. But a lot of religion begins with the idea that the world is real and that the actions of human beings matter, whether you are good in some way. I think that is actually a fairly common starting point. After that I think that there are differences on deciding who is in charge of the order, let’s say. But I think they start off from some similar things. What was the rest of the question?
There is the difference when you are thinking about religion you are thinking also about the postulates and the religious beliefs that you can’t question. Are there some dogmas in science that you just not mention?
Roald Hoffman: One can look for dogmas in science and I think they are there. At the same time the edible complex is very strong in science of young people making careers by slaying their fathers, by drawing out ideas and that is an accepted way of doing so. A much less respect for authority in the end. But there are dogmas. So why are there dogmas? Because there are human beings at work.
But somehow the social system of science that there are many people doing things assures that in the long run those dogmas do not stay dogmas. Whereas the social system of religion perpetuates the authority often. And makes it more difficult to introduce change. Interesting balance between change and stasis in science. You do invoke authority that is in part what those footnotes are about in papers, but at the same time you want to do something new. An interesting system.
So we are somewhere near the question of the truth. You mentioned that science is not searching for truth.
Roald Hoffman: I think it is searching for approximations to the truth but I tell you why I don’t want us to be put up in a certain 19th century way as searchers for the truth. First of all we all know that there are different approaches to the truth and the truth as a femoral can be expressed in different words, even if we all believe there is an underlying reality, and I do believe that. But I think our representations of that reality are human made. In that sense I also think that religions are human made. Even though there are something that seeks, I think religion is an emergent phenomenon like science and like … and like literature it is human.
The reason I do not want us to be searching after truth is we put ourselves up then as, now we will mix the metaphors of religion and science as priests of the truth and then we have that much further to fall. Why is the public so interested in fraud and science? For the same reasons it is interested in the sexual misdeeds of our ministers. You set yourself up ethically at some point to be about truth or morals and then you do not obey it, then people take delight in that somehow, because it confirms to them they are all members of weakness in some way, that they can not be good all the time. And so I think fraud and science is not important actually, because of the system of science and also I think because of the psychopathology of fraud.
Let me explain. I think the people who forge data or facts are usually sick in someway. And being sick there are two consequences. One is that there are some natural defences against thinking that they will be caught when they forge are abrogated, are put aside so they do crazy things. Second is that they never forged anything dull, they always forge something interesting. For instance even in the most devious cases there is some student who is sick and there is some professor who has a favourite theory and a student concocts an experiment which supports that theory. Something like this happened at Cornell. But it is always something interesting. It is not a little everyday cheating of that type. And so given that scientists, being human, are much more likely to try to prove somebody wrong than to prove somebody right. The moment somebody comes out with something interesting sure enough there is somebody around the world who has some vested interest in that problem, an alternative theory who will test that experiment.
But about that question of truth, you mentioned that there is this part about complex in science, that you always have to throw away the old truths and prove the new ones. So it is something about the provisoric nature of the truth and science.
The truth is provisional. It is to be modified in different ways. I think reliable knowledge is, that phrase reliable knowledge that I like is not mine, I borrowed it from John Ziman who is a British physicist and sociologist of science has written about this. I sort of like it. I think we try to get things that are reasonably reproducible and that can be used. And they can be. The description for an aspirin factory written in Japanese or in Portuguese allows that factory to be built independent of the language that it is written in.
There is something else there also. People talk about the relation between the truth, beauty and the simplicity. Do you think there is such a relation?
Roald Hoffman: No. I think this is a falling into the weakness of the human mind to think that the world is simple. And it is an interesting tripartite relationship. I guess I’m fighting in part what I would say is, pernicious ideas of physicists coming into chemistry who think the moment you can write up Sherman’s equation that all of chemistry is solved. And behind that are statements by the Iraq great physicist, theoretical physicist about this equation that an equation, if it is beautiful, must be true.
Yes, some are in the old world but not in this world that we have. The equations are simple in part and yet complex. Symmetry is there, symmetry gets broken. Things happen in a body especially when they are subject to evolution things in incredibly complicated ways. The way a message is sent from one nerve to another with an intermediate of a molecule, dopamine on serotonin and there is one thing that sets it loose and another one that detects it, but it is not done in one way. After a while this thing looks like what we would call in the United States, a Rube Goldberg machine, one of those cartoons where there is something that does something else that does something else and terribly complicated.
So those things are I think those are natural, that things are complicated. I think it is the dreams of the simplicity of our mind which cannot sometimes be over complexity and that’s true of personal relationships as well as it is with politics or with science. What is interesting to me is that scientists should fall for simplicity. Given that they are faced with the realities of complexity all the time. And they will come back and tell you, physicists, well if you take it apart underneath there are some simple equations. Yes, but the taking apart has destroyed the essence and the reality of anything that is real, and they never put it together. They only take the watch apart like children.
So this is reductionism?
Roald Hoffman: Yes.
Is this the voice of a chemist?
Roald Hoffman: Yes. It’s a voice of a chemist and the voice of someone trying to fight reductionism, exemplified by what Stephen Weinberg writes about or other people like that. And it is in defence of the complexity that is the reality of the real world. I think it is also perhaps a poet talking here and wants to see the particular and sometimes does not want things taken apart. Both the particular and the relationship with the whole to each other. There are sciences that look at that but not the ideology of science, the philosophy of science because the philosophers who did this, with one exception of Michael Polanyi, were a physicist in that training or their training was in logic in philosophy tend to go for the simplistic point of view.
So as you mentioned this is also a voice of a poet, not only a chemist so maybe we can listen to one of your poems?
Roald Hoffman: Sure. Let me read a poem but let me preface it by some comments about it. In it you will see many voices of different ways of trying to understand the world around something simple which is something happening in Provence in France, […]. And the poem is called ‘Enough Already’ and behind it, initially it was called ‘Dayenu’ which is what one a prayer, a song in the Passover say there, which means that would have been enough if God had only taken us out of Israel that would have been enough, but he had done other things. And there is a little bit of that. And there is in this also … so there is something about my Jewish background in this, in the phraseology here. Let me read it anyway.
Enough Already
You walk in to the sun-
splashed olives’ mossy
trunks, greener than
fresh grass. This doesn’t
seem to be enough
so you think – even
here they grow olives
only on warm terraces;
and ask who first found
olives had to be cured?
This cleverness, too
does not satisfy. So,
walking hand-in-hand
into the grove you say:
the world needs us
(and other lovers)
to give such life; which
would do nicely for most,
save those who’d leave it
for a Creator. But
then, alone, you look
real close, and the black
spot on the green bark
you reach for sharpens
into inch-and-a-half of
scorpion, you see a
red beetle, and by God,
that does suffice.
Thank you.
Roald Hoffman: So there is not too much science in that poem.
No. It is good. Do you find science is an inspiration for your writing?
Roald Hoffman: Yes, it is. It is limited because some of the sort of poetry and sciences depend on them being part of the cognitive framework. Knowing what went before, what it is about. But part of it is what I find inspiring is the language, the kind of natural language that scientists speak, and they don’t the language is important. They think equations and chemical formulas are important. But language is all we’ve got, and this language is made to serve and express things so words like energy and force mean special things well defined and we try to make them fit. And then there are words invented and words used, let me give you an example.
What often happens to me is it is given to me to go to dull seminars and sometimes after I fall asleep I wake up and it is still going on and so I listen sometimes to the words even if they are not so interesting. And someone came at a seminar and was talking about some mathematical equation, he said “Let us assume free boundary conditions”. He was talking about some technical words, boundary conditions are what you do on a mathematical equation to make it fit at the ends but sort of the idea, free boundaries was very interesting because it was a typical Zen idea of something being free and something being constrained. So I worked that into a poem. So the language is interesting. Then there is a lot of metaphor, let me give you an example.
In science and in poetry?
Roald Hoffman: Yes, in science which I can use in poetry. Someone was talking about once at another seminar about the metamict state. It is a funny word, metamict. It is not used very much. Someone had invented it, so what was it? It was a state of matter of, for instance, radioactive minerals. Minerals in which there was a radioactive atom like thorium. The minerals when formed gave beautiful transparent crystals, perfect crystals, but after a while the atoms which were sitting at the sites in the crystal began decomposing in nuclear reactions. And as they emitted some ray they rebounded and the ions that were left behind bounced into other atoms and created this order in the lattice and with time the crystal grew whitish yellow and amorphous dull looking and was destroyed. And that was the metamict state. It’s a poem by itself, I mean the ending is within that. And all that order has been destroyed without any plan to do so. So I took that and wrote a poem about that. So there are metaphors like that.
Yes. I wonder what gives you satisfaction.
Roald Hoffman: It is still all of the things, I should let go of some of them. On all means the science and the teaching and the writing. They all give tremendous satisfaction. I’m not good at anything else like music so it is the writing through which I exercise my literary artistic activities. They are all tied together somehow because what gives me the greatest pleasure in the research is finding an explanation which to me is teaching. Or seeing some relationship between two things. Like that there is a group of atoms in inorganic chemistry that behaves in a similar way to organic chemist, to an organic grouping. That a manganese with five carbon monoxides attached to it is like a methyl group like a CH3. Now I see that as both poetry and teaching because I’ve made a connection between organic and inorganic. In some way I have created a metaphor. This like that and that is a little bit like a poem.
I’ve also used that for teaching. I give it out to the community, I publish it and I talk about it. And it also still gives me satisfaction to teach first year chemistry which this last year, the year 2003, 2004, I taught two first year courses, usually I teach one first year as I’m doing this year. You know I’ve taught that material for 40 years, I know it pretty well but first of all I find some new ways to say it, unfortunately it stretches too long now. Second, there is a light in the kids’ eyes, not everyone, some of them they are asleep and for some of them are too driven by professional aims, society and their parents and themselves. Let’s not blame just the society, to become doctors or engineers and they don’t think enough. But some of them just relax and I see, I know because I am a good teacher, the nonverbal science that they’ve understood. And I’ve released something in their minds. I haven’t, the facts that I’ve told them are irrelevant, I’ve empowered them to think, that is a wonderful feeling.
It sounds like the key to creativity that you are talking about is that you all the time go back to the start point somehow, to the first great students and the first questions.
Roald Hoffman: It has been important to me. I’ve been lucky that I’m doing theory. So theory is about explaining and understanding phenomenon. Therefore I’m already in a quasi-teaching mode. Then I also have found that I am good at explaining to experimental chemists complicated theoretical things. One thing I’ve done since the Nobel Prize which has been important to me is the simple teaching thing within research that is I have explained to a community of chemists called inorganic /—/ chemists, how not to be afraid of the language of physics. Language of band structures and densities of states of firming levels. The kind of thing that is involved in designing new super conductors. And I wrote a book about it but I have also been writing many articles, actually mostly I write articles. By little examples I have been able to teach chemists not to be afraid of this language. I have done some good things along the way too but I think it is that teaching thing which has given me pleasure.
You have done a lot of interesting research and written poems and written essays and also a play and you are even behind the scientific café in Greenwich Village in the university. And you have also been participating in a carnival, last year’s carnival in the Rio de Janeiro.
Roald Hoffman: I helped a little, yes. Actually, went down to carnival for a science. Hard to believe.
So my question would be how do your colleagues or the scientific community react to that?
Roald Hoffman: Well, I think or how do they react to the poetry in general to the excursions out of science. On one hand they react positively, and they are glad someone is doing it. They have a lot of respect for spiritual things. It shows up in interesting ways not necessarily directly, but it shows up in little things like when somebody writes a book that they use a poem as an epigraph or something like that. I think some of them think that he can afford to do it and we can’t and to some extent they are right.
The young assistant professor up for promotion, should he or she put up front their activity outside the science. I think they should put in something about their teaching and their attempts to communicate to the public. But very often they are afraid to do so and they make some judgement and it is correct that it is not the prime thing they are evalued on but it certainly adds little on the edges and adds some colour, could make a difference in a decision. Some people are just, some of my colleagues might think that this is something one does when one no longer does science, goes into administration, does history, sociology of science. Well, so to them I have to prove to them that I still do science. I have just finished writing two proposals. Maybe I feel I have to prove but I enjoy doing it also.
You know what hurts the most is when it comes through that somehow people think that this activity of writing poetry or running this café or something, when they think this is easy. That hurts. Because on a poem I go through typically 15, 20 drafts, I don’t do that on a scientific paper. No, it is true. I have written 500 scientific papers, so it becomes easier but still it was always easier to do the science and I am actually trying something harder. And they just have to try it, I would say. So I get angry at that if that happens. But by and large there is a lot of respect in the community for this.
The poetry writing, does this make you a better scientist do you think?
Roald Hoffman: I think the poetry writing of course makes me feel better about myself for having tried to do that. I think that is true about anyone, that they need some other thing to do. I think the poetry writing has given me a bit of an appreciation and more for the power of concise statement but then I have that in science too. Once again comes back maybe simplicity, but it is not quite simplicity because it is a complex statement of word but it says something economically. Something intensely. And I have learned a little how to do that through the poetry.
I think much more than either the science helping me to do the poetry or the poetry helping the science. I think I am working out something that is in me that wants to see the relationships, to watch to see relationships, to communicate that to people. And I am doing that in the science and I am doing that in the poetry in other ways. And occasionally because I don’t believe in separating my worlds I will separate them. I’ll not write that article or the poetry because I am not going to get it published because I have got to get by the gate keepers. But occasionally and the kind of thing I have done here in Stockholm, this visit, someone gives me a chance to talk about both worlds and not to separate them and I love it.
So my final question would be how do you find time to do all of this?
Roald Hoffman: Oh, that is not a good question because it is, I’ll answer it honestly but there is no time to do all this. First of all, I should probably give up the science and do the writing, so why should I? If I had some rational reasoning behind it but I’m not rational. The rational reasoning is that there is simply lots of good people doing the science, there are fewer people and therefore I think it is of more value to society to be able to work on this area in-between, of popularising science, of building bridges between the scientists and especially the shapers of the spirit and the arts and humanities. There are less people doing that. But I still find the science fun, first of all.
But now how do I do all this? So I haven’t given up the science and the teaching. I am 67 and I could retire but I don’t want to, I don’t have to, so I am still doing the science. As I said I have just written two major research proposals to the National Science Foundation for two different aspects of my work, each with three years of research. I have ideas that I want to work them out. So I have added on the writing to the science and I would say that if I were to analyse my days are very complex and interesting. But if I were to analyse it more than half the time I spend probably on writing or societal activities somehow related to it more than on the science. I’ve added it on and it has not been done without personal problems in my life. It is not easy to live with someone like me who does all these things because I need to be in control of my time, I need to be able to, if I want to, to sit down and write and there have been stresses in my personal life as a result. So, it has not been without some difficulties.
This is the press?
Roald Hoffman: Yes.
I wish we had much more time to talk, I am afraid we have to conclude. Thank you so much, Professor Hoffman.
Did you find any typos in this text? We would appreciate your assistance in identifying any errors and to let us know. Thank you for taking the time to report the errors by sending us an e-mail.
Roald Hoffmann – Other resources
Links to other sites
Roald Hoffman’s page at Cornell University
Roald Hoffmann’s personal website
Roald Hoffmann – Facts
Roald Hoffmann – Biographical

I came to a happy Jewish family in dark days in Europe. On July 18, 1937 I was born to Clara (née Rosen) and Hillel Safran in Zloczow, Poland. This town, typical of the Pale of the Settlement, was part of Austria-Hungary when my parents were born. It was Poland in my time and is part of the Soviet Union now. I was named after Roald Amundsen, my first Scandinavian connection. My father was a civil engineer, educated at the Lvov (Lemberg) Polytechnic, my mother by training a school teacher.
In 1939 the war began. Our part of Poland was under Russian occupation from 1939-1941. Then in 1941 darkness descended, and the annihilation of Polish Jewry began. We went to a ghetto, then a labor camp. My father smuggled my mother and me out of the camp in early 1943, and for the remainder of the war we were hidden by a good Ukrainian in the attic of a school house in a nearby village. My father remained behind in the camp. He organized a breakout attempt which was discovered. Hillel Safran was killed by the Nazis and their helpers in June 1943. Most of the rest of my family suffered a similar fate. My mother and I, and a handful of relatives, survived. We were freed by the Red Army in June 1944. At the end of 1944 we moved to Przemysl and then to Krakow, where I finally went to school. My mother remarried, and Paul Hoffmann was a kind and gentle father to me until his death, two months prior to the Nobel Prize announcement.
In 1946 we left Poland for Czechoslovakia. From there we moved to a displaced persons’ camp, Bindermichl, near Linz, in Austria. In 1947 we went on to another camp in Wasseralfingen bei Aalen in Germany, then to München. On Washington’s Birthday 1949 we came to the United States.
I learned English, my sixth language at this point, quite quickly. After P.S. 93 and P.S. 16, Brooklyn, I went on to the great Stuyvesant High School, one of New York’s selective science schools. Among my classmates were not only future scientists but lawyers, historians, writers – a remarkable group of boys. In the summers I went to Camp Juvenile in the Catskills, a formative experience. Elinor, my younger sister, was born in 1954.
In 1955 I began at Columbia College as a premedical student. That summer and the next I worked at the National Bureau of Standards in Washington with E.S. Newman and R.E. Ferguson. The summer after I worked at Brookhaven National Laboratory, with J.P. Cumming. These summers were important because they introduced me to the joys of research, and kept me going through some routine courses at Columbia. I did have some good chemistry teachers, G.K. Fracnkel and R.S. Halford, and a superb teaching assistant, R. Schneider. But I must say that the world that opened up before me in my non science courses is what I remember best from my Columbia days. I almost switched to art history.
In 1958 I began graduate work at Harvard. I intended to work with W.E. Moffitt, a remarkable young theoretician, but he died in my first year there. A young instructor, M.P. Gouterman, was one of the few faculty members at Harvard who at that time was interested in doing theoretical work, and I began research with him. In the summer of 1959 I got a scholarship from P.O. Lowdin’s Quantum Chemistry Group at Uppsala to attend a Summer School. The school was held on Lidingö, an island outside of Stockholm. I met Eva Börjesson who had a summer job as a receptionist at the school, and we were married the following year.
I came back to Harvard, began some abortive (and explosive) experimental work, and Eva and I took off for a year to the Soviet Union. It was the second year of the U.S.-U.S.S.R graduate student exchange. I worked for 9 months at Moscow University with A.S. Davydov on excitor theory. Eva and I lived in one of the wings, Zona E, of that great central building of Moscow University. My proficiency in Russian and interest in Russian culture date from that time.
On returning to the U.S. I switched research advisors and started to work with W.N. Lipscomb, who had just come to Harvard. Computers were just coming into use. With Lipscomb’s encouragement and ebullient guidance, L.L. Lohr and I programmed what was eventually called the extended Hückel method. I applied it to boron hydrides and polyhedral molecules in general. One day I discovered that one could get the barrier to internal rotation in ethane approximately right using this method. This was the beginning of my work on organic molecules.
In 1962 I received my doctorate, as the first Harvard Ph.D. of both Lipscomb and Gouterman. Several academic jobs were available, and I was also offered a Junior Fellowship in the Society of Fellows at Harvard. I chose the Junior Fellowship. The three ensuing years in the Society (1962 – 65), gave me the time to switch my interests from theory to applied theory, specifically to organic chemistry. It was EJ. Corey who taught me, by example, what was exciting in organic chemistry. I began to look at all kinds of organic transformations, and so I was prepared when in the Spring of 1964 R.B. Woodward asked me some questions about what subsequently came to be called electrocyclic reactions. That last year at Harvard was exciting. I was learning organic chemistry at a great pace, and I had gained access to a superior mind. R.B. Woodward possessed clarity of thought, powers of concentration, encyclopedic knowledge of chemistry, and an aesthetic sense unparalleled in modern chemistry. He taught me, and I have taught others.
The 1962 – 65 period was creative in other ways as well: Our two children, Hillel Jan and Ingrid Helena, were born to Eva and me.
In 1965 I came to Cornell where I have been ever since. A collegial department, a great university and a lovely community have kept me happy. I am now the John A. Newman Professor of Physical Science. I have received many of the honors of my profession. I am especially proud that in addition to the American Chemical Society’s A.C. Cope Award in Organic Chemistry, which I received jointly with R.B. Woodward in 1973, I have just been selected for the Society’s Award in Inorganic Chemistry in 1982, the only person to receive these two awards in different subfields of our science.
I have been asked to summarize my contributions to science.
My research interests are in the electronic structure of stable and unstable molecules, and of transition states in reactions. I apply a variety of computational methods, semiempirical and nonempirical, as well as qualitative arguments, to problems of structure and reactivity of both organic and inorganic molecules of medium size. My first major contribution was the development of the extended Huckel method, a molecular orbital scheme which allowed the calculation of the approximate sigma- and pie- electronic structure of molecules, and which gave reasonable predictions of molecular conformations and simple potential surfaces. These calculations were instrumental in a renaissance of interest in sigma electrons and their properties. My second major contribution was a two-pronged exploration of the electronic structure of transition states and intermediates in organic reactions. In a fruitful collaboration R.B. Woodward and I applied simple but powerful arguments of symmetry and bonding to the analysis of concerted reactions. These considerations have been of remarkable predictive value and have stimulated much productive experimental work. In the second approach I have analyzed, with the aid of various semiempirical methods, the molecular orbitals of most types of reactive intermediates in organic chemistry-carbonium ions, diradicals, methylenes, benzynes, etc.
Recently I and my collaborators have been exploring the structure and reactivity of inorganic and organometallic molecules. Approximate molecular orbital calculations and symmetry-based arguments have been applied by my research group to explore the basic structural features of every kind of inorganic molecule, from complexes of small diatomics to clusters containing several transition metal atoms. A particularly useful theoretical device, the conceptual construction of complex molecules from MLn fragments, has been used by my research group to analyze cluster bonding and the equilibrium geometries and conformational preferences of olefin and polyene metal carbonyl complexes. A satisfactory understanding of the mode of binding of essentially every ligand to a metal is now available, and a beginning has been made toward understanding organometallic reactivity with the exploration of potential energy surfaces for ethylene insertion, reductive elimination and alkyl migrative insertion reactions. Several new structural types, such as the triple-decker and porphyrin sandwiches, have been predicted, and recently synthesized by others. On the more inorganic side, we have systematically explored the geometries, polytopal rearrangement and substitution site preferences of five, six, seven and eight coordination, the factors that influence whether certain ligands will bridge or not, the constraints of metal-metal bonding, and the geometry of uranyl and other actinide complexes. I and my coworkers are beginning work on extended solid state structures and the design of novel conducting systems.
The technical description above does not communicate what I think is my major contribution. I am a teacher, and I am proud of it. At Cornell University I have taught primarily undergraduates, and indeed almost every year since 1966 have taught first-year general chemistry. I have also taught chemistry courses to non-scientists and graduate courses in bonding theory and quantum mechanics. To the chemistry community at large, to my fellow scientists, I have tried to teach “applied theoretical chemistry”: a special blend of computations stimulated by experiment and coupled to the construction of general models – frameworks for understanding.
This autobiography/biography was written at the time of the award and later published in the book series Les Prix Nobel/ Nobel Lectures/The Nobel Prizes. The information is sometimes updated with an addendum submitted by the Laureate.
Added in 1992
In the last decade I and my coworkers have begun to look at the electronic structure of extended systems in one-, two-, and three dimensions. Frontier orbital arguments find an analogue in this work, in densities of states and their partitioning. We have introduced an especially useful tool, the COOP curve. This is the solid state analogue of an overlap population, showing the way the bond strength depends on electron count. My group has studied molecules as diverse as the platinocyanides, Chevrel phases, transition metal carbides, displacive transitions in NiAs, MnP and NiP, new metallic forms of carbon, the making and breaking of bonds in the solid state and many other systems. One focus of the solid state work has been on surfaces, especially on the interaction of CH4 , acetylene and CO with specific metal faces. The group has been able to carry through unique comparisons of inorganic and surface reactions. And in a book “Solids and Surfaces. A Chemist’s View of Bonding in Extended Structures,” I’ve tried to teach the chemical community just how simple the concepts of solid state physics are. And, a much harder task, to convince physicists that there is value in chemical ways of thinking.
In 1986-88 I participated in the production of a television course in introductory chemistry. “The World of Chemistry” is a series of 26 half-hour episodes developed at the University of Maryland and produced by Richard Thomas. The project has been funded by Annenberg/the Corporation for Public Broadcasting. I am the Presenter for the series which began to be aired on PBS in 1990, and will also be seen in many other countries.
My first real introduction to poetry came at Columbia from Mark Van Doren, the great teacher and critic whose influence was at its height in the 1950’s. Through the years I maintained an interest in literature, particularly German and Russian literature. I began to write poetry in the mid-seventies, but it was only in 1984 that a poem was first published. I own much to a poetry group at Cornell that includes A.R. Ammons, Phyllis Janowitz and David Burak, as well as to Maxine Kumin. My poems have appeared in many magazines and have been translated into French, Portuguese, Russian and Swedish. My first collection, “The Metamict State”, was published by the University of Central Florida Press in 1987, and is now in a second printing. A second collection, “Gaps and Verges”, was also published by the University of Central Florida Press, in 1990. Articles on my poetry have appeared in Literaturnaya Gazeta and Studies in American Jewish Literature. I received the 1988 Pergamon Press Fellowship in Literature at the Djerassi Foundation, Woodside, California, where I was in residence for three years.
It seems obvious to me to use words as best as I can in teaching myself and my coworkers. Some call that research. Or to instruct others in what I’ve learned myself, in ever-widening circles of audience. Some call that teaching. The words are important in science, as much as we might deny it, as much as we might claim that they just represent some underlying material reality.
It seems equally obvious to me that I should marshal words to try to write poetry. I write poetry to penetrate the world around me, and to comprehend my reactions to it.
Some of the poems are about science, some not. I don’t stress the science poems over the others because science is only one part of my life. Yet there are several reasons to welcome more poetry that deals with science.
Around the time of the Industrial Revolution – perhaps in reaction to it, perhaps for other reasons – science and its language left poetry. Nature and the personal became the main playground of the poet. That’s too bad for both scientists and poets, but it leaves lots of open ground for those of us who can move between the two. If one can write poetry about being a lumberjack, why not about being a scientist? It’s experience, a way of life. It’s exciting.
The language of science is a language under stress. Words are being made to describe things that seem indescribable in words – equations, chemical structures and so forth. Words do not, cannot mean all that they stand for, yet they are all we have to describe experience. By being a natural language under tension, the language of science is inherently poetic. There is metaphor aplenty in science. Emotions emerge shaped as states of matter and more interestingly, matter acts out what goes on in the soul.
One thing is certainly not true: that scientists have some greater insight into the workings of nature than poets. Interestingly, I find that many humanists deep down feel that scientists have such inner knowledge that is barred to them. Perhaps we scientists do, but in such carefully circumscribed pieces of the universe! Poetry soars, all around the tangible, in deep dark, through a world we reveal and make.
It should be said that building a career in poetry is much harder than in science. In the best chemical journal in the world the acceptance rate for full articles is 65%, for communications 35%. In a routine literary journal, far from the best, the acceptance rate for poems is below 5%.
Writing, “the message that abandons”, has become increasingly important to me. I expect to publish four books for a general or literary audience in the next few years. Science will figure in these, but only as a part, a vital part, of the risky enterprise of being human.
Kenichi Fukui – Banquet speech
Kenichi Fukui’s speech at the Nobel Banquet, December 10, 1981
Your Majesties, Your Royal Highnesses, Ladies and Gentlemen,
I have the privilege to speak on behalf of Professor Roald Hoffmann and myself and to express first of all our deepest gratitude for the high honour and warm hospitality that have been given us on this occasion.
Chemistry itself knows altogether too well that – given the real fear that the scarcity of global resources and energy might threaten the unity of mankind – chemistry is in a position to make a contribution towards securing a true peace on earth.
We pray that every field of science may contribute in bringing happiness – not disaster – to human beings. In that spirit we wish to accept this highest honour – in the cause of peace – not for ourselves alone, but for all researchers in basic chemistry. In particular, for younger researchers on whom the future of mankind may depend. We believe that they are working with all the scientific wisdom at their disposal for the preservation of the inheritance of the earth and for the lasting survival of mankind.
Kagaku no kenkyu no oyo ni oite nani ga zen de soshite – moshimo arutosureba – nani ga aku de aruka o mottomo yoku miwakerunowa kagaku no sentanteki na ryoiki ni hataraku mottomo sugureta kagakushatachi desu. (What I said in Japanese means: We think that it is the best scientists working in the frontier fields of science who are best able to judge what is good and what is bad – if any – in the application of their scientific research).
Thank you very much.
The Nobel Prize in Chemistry: The development of modern chemistry
by Bo G. Malmström and Bertil Andersson*
1. Introduction
1.1 Chemistry at the borders to physics and biology
The turn of the century 1900 was also a turning point in the history of chemistry. Consequently, a survey of the Nobel Prizes in Chemistry during this century will provide an analysis of important trends in the development of this branch of the Natural Sciences, and this is the aim of the present essay. Chemistry has a position in the center of the sciences, bordering onto physics, which provides its theoretical foundation, on one side, and onto biology on the other, living organisms being the most complex of all chemical systems. Thus, the fact that chemistry flourished during the beginning of the 20th century is intimately connected with fundamental developments in physics.
In 1897 Sir Joseph John Thomson of Cambridge announced his discovery of the electron, for which he was awarded the Nobel Prize for Physics in 1906. He found that these negatively charged ‘corpuscles’, as he called them, have a mass 1000 times smaller than the hydrogen atom. Thomson’s discovery had, of course, important implications for chemistry, as it showed that the atom is not an indivisible building block of chemical compounds, but it took a number of years before this led to developments of direct relevance to chemistry. In 1911 Ernest Rutherford, who had worked in Thomson’s laboratory in the 1890s, formulated an atomic model, according to which the positively charged atomic nucleus carries most of the mass of the atom but occupies a very small part of its volume.
This is instead created by a cloud of electrons circling around the nucleus. Rutherford received the Nobel Prize for Chemistry already in 1908 for his work on radioactivity (see Section 2).
It was soon realized that in Rutherford’s atomic model the stability of atoms was at variance with the laws of classical physics, since the electrons would lose energy in the form of electromagnetic radiation and eventually fall into the nucleus. Niels Bohr from Copenhagen understood that an important clue to the solution of this problem could be found in the distinct lines observed in the spectra of atoms, the regularities of which had been discovered in 1890 by the physics professor Johannes (Janne) Rydberg at Lund University. Consequently, Bohr formulated in 1913 an alternative atomic model, in which only certain circular orbits of the electrons are allowed. In this model light is emitted (or absorbed), when an electron makes a transition from one orbit to another. Bohr received the Nobel Prize for Physics in 1922 for his work on the structure of atoms.
Another step in the application of the electronic structure of atoms to chemistry was taken in 1916, when Gilbert Newton Lewis suggested that strong (covalent) bonds between atoms involve a sharing of two electrons between these atoms (electron-pair bond). Lewis also contributed fundamental work in chemical thermodynamics, and his brilliant textbook, Thermodynamics (1923), written together with Merle Randall, is counted as one of the masterworks in the chemical literature. Much to the surprise of the chemical community, Lewis never received a Nobel Prize.
Even if the contributions just described were made a decade or more after Thomson’s discovery, much important work in the borderland between physics and chemistry was published in the 1890s, and this was naturally given a strong consideration by the first Nobel Committee for Chemistry (see Section 2). In fact, three of the Laureates during the first decade, Jacobus Henricus van’t Hoff, Svante Arrhenius and Wilhelm Ostwald, are generally regarded as the founders of a new branch of chemistry, physical chemistry. Fundamental work had, however, also been done in more traditional chemical fields, particularly in organic chemistry and in the chemistry of natural products, which is clearly reflected in the early prizes. The Nobel Committee, in addition, showed great openness and foresight by recognizing the other border, that towards biology, already in 1907 with the prize to Eduard Buchner “for his biochemical researches and his discovery of cell-free fermentation”.
1.2 The mechanics of the work in the Nobel committee for chemistry
According to the statutes of the Nobel Foundation, the Nobel Committees should have five members, but the Committee for Chemistry has in recent decades chosen to widen its expertise by adding a number of adjunct members (five in 1998) with the same voting rights as the regular members. Until recently there was no limit other than age on how many times regular members could be re-elected for 3-year terms, so that some members sat on the Committee for a very long period. For example, Professor Arne Westgren of Stockholm, who was secretary of the Nobel Committees for Physics and for Chemistry 1926-1943, was also Chairman of the Committee for Chemistry 1944-1965. Present rules, however, only allow two re-elections, so that a member’s maximum total time on the Committee will be nine years.
Only persons that have been properly nominated before 31 January can be considered for the Nobel Prize in a given year. Consequently, the Nobel Committee starts its work by sending out invitations to nominate in the autumn of the preceding year. Recipients of these invitations, for both Physics and Chemistry, are: 1) Swedish and foreign members of the Royal Swedish Academy of Sciences; 2) members of the Nobel Committees for Physics and for Chemistry; 3) Nobel Laureates in Physics and Chemistry; 4) professors in Physics and Chemistry in Scandinavian universities and at Karolinska Institutet; 5) professors in these subjects in a number of universities outside Scandinavia, selected on a rotation basis by the Academy of Sciences; and 6) other scientists that the Academy chooses to invite.
In the initial years of the Nobel Prize, about 300 invitations to nominate for the Nobel Prize for Chemistry were sent out, but this number has increased over the years and was as high as 2,650 in 1998. The number of nominations received has also increased dramatically from 20-40 during the first decade to 400-500 in the 1990s. The number of candidates is usually smaller than the number of nominations, since many candidates receive more than one nomination. During the first few years only about 10 scientists were nominated, but in recent years this number has been in the range of 250-350.
The invitations to nominate are personal, and it is stressed that nominations should not be discussed with the candidate or with colleagues. This is unfortunately not always respected as is obvious from the fact that many identically worded nominations are some years received from the same university. For this reason the Committee does not put much weight on the number of nominations a given candidate receives, unless clearly independent nominations come from different universities in different countries. This attitude was not taken in earlier years however, as is evident from the following statement made by Committee Chairman Arne Westgren, in a survey over the first 60 years of the Nobel Prize for Chemistry [1]: “In fact, if a scientist is proposed by a large number of sponsors in the preliminary international voting, he is normally selected by the Academy.”
Often the same candidate receives nominations both for chemistry and for physics or for chemistry and for medicine. This problem was met already in 1903, when Arrhenius had been nominated both for the Prize for Chemistry and that for Physics, and in its deliberations the Committee for Chemistry suggested that he should be awarded half of each Prize, but this idea was rejected by the Committee for Physics. Because of such borderline problems, the Committee for Chemistry nowadays has joint meetings with those for Physics and for Physiology or Medicine. However, as pronounced by Westgren [1]: “It is now generally recognized that the important thing is to decide whether work which can with equal justice be reckoned as chemistry and physics or chemistry and medicine, is in fact worthy of a Nobel Prize.” For example, Peter Mitchell, who received the 1978 Nobel Prize for Chemistry, could with equal justice have been awarded the Prize for Physiology or Medicine.
Nobel’s will laid down that the prize should be awarded for work done during the preceding year, but in the statutes governing the committee work this has been interpreted to mean the most recent results, or for older work provided its significance has only recently been demonstrated. It was undoubtedly this rule that excluded Stanislao Cannizzaro from receiving one of the first Nobel Prizes, since his work on drawing up a reliable table of atomic weights, helping to establish the periodic system, was done in the middle of the 19th century. A more recent example is Henry Eyring, whose brilliant theory for the rates of chemical reactions, published in 1935, was apparently not understood by members of the Nobel Committee until much later. As a compensation the Royal Swedish Academy of Sciences gave him, in 1977, its highest honor, other than the Nobel Prize, the Berzelius Medal in gold.
2. The first decade of Nobel Prizes for Chemistry
So much fundamental work in chemistry had been carried out during the last two decades of the 19th century that, as stated by Westgren [1], “During the first few years the Academy was chiefly faced with merely deciding the order in which these scientists should be awarded the prize.” For the first prize in 1901 the Academy had to consider 20 nominations, but no less than 11 of these named van’t Hoff, who was also chosen by the Committee for Chemistry. van’t Hoff had already during his thesis work in Utrecht in 1874 published his suggestion that the carbon atom has its four valences directed towards the corners of a regular tetrahedron, a concept which is the very foundation of modern organic chemistry. The Nobel Prize was, however, awarded for his later work on chemical kinetics and equilibria and on the osmotic pressure in solution, published in 1884 and 1886, when he held a professorship in Amsterdam. When he received the prize he had, however, left this for a position at Akademie der Wissenschaften in Berlin in 1896.
In his 1886 work van’t Hoff showed that most dissolved chemical compounds give an osmotic pressure equal to the gas pressure they would have exerted in the absence of the solvent. An apparent exception was aqueous solutions of electrolytes (acids, bases and their salts), but in the following year Arrhenius showed that this anomaly could be explained, if it is assumed that electrolytes in water dissociate into ions. Arrhenius had already presented the rudiments of his dissociation theory in his doctoral thesis, which was defended in Uppsala in 1884 and was not entirely well received by the faculty. It was, however, strongly supported by Ostwald in Riga, who, in fact, travelled to Uppsala to initiate a collaboration with Arrhenius. In 1886-1890 Arrhenius did work with Ostwald, first in Riga and then in Leipzig, and also with van’t Hoff in Berlin. When Arrhenius was awarded the Nobel Prize for Chemistry in 1903, he was since 1895 professor of physics in Stockholm, and he was also nominated for the Prize for Physics (see Section 1).
The award of the Nobel Prize for Chemistry in 1909 to Ostwald was chiefly in recognition of his work on catalysis and the rates of chemical reactions. Ostwald had in his investigations, following up observations in his thesis in 1878, shown that the rate of acid-catalyzed reactions is proportional to the square of the strength of the acid, as measured by titration with base. His work offered support not only to Arrhenius’ theory of dissociation but also to van’t Hoff’s theory for osmotic pressure. Ostwald was founder and editor of Zeitschrift für Physikalische Chemie, the publication of which is generally regarded as the birth of this new branch of chemistry.
Three of the Nobel Prizes for Chemistry during the first decade were awarded for pioneering work in organic chemistry. In 1902 Emil Fischer, then in Berlin, was given the prize for “his work on sugar and purine syntheses”. Fischer’s work is an example of the growing interest from organic chemists in biologically important substances, thus laying the foundation for the development of biochemistry, and at the time of the award Fischer mainly devoted himself to the study of proteins. Another major influence from organic chemistry was the development of chemical industry, and a chief contributor here was Fischer’s teacher, Adolf von Baeyer in Munich, who was awarded the prize in 1905 “in recognition of his services in the advancement of organic chemistry and the chemical industry, … .” His contributions include, in particular, structure determination of organic dyes (indigo, eosin) and the study of aromatic compounds (terpenes). The third Laureate working in organic chemistry was Otto Wallach in Göttingen, who, like von Baeyer, contributed to alicyclic chemistry, studying not only terpenes but also camphor and other components of ethereal oils. At the award ceremony in 1910 the importance of his discoveries for chemical industry was emphasized.
Two of the early prizes were given for the discovery of new chemical elements. Sir William Ramsay from London received the 1904 Nobel Prize for Chemistry for his discovery of a number of noble gases, a new group of chemically unreactive elements. The first one isolated was argon (“the inactive one”), which Ramsay discovered in 1894, in collaboration with Lord Rayleigh [John William Strutt Rayleigh] of the Royal Institution, who was awarded the Prize for Physics in the same year, his investigations of the density of air and other gases forming the basis for this discovery. The following year Ramsay found helium, observed earlier only in the solar spectrum (hence its name), in emanations from radium, thus anticipating later prizes for nuclear chemistry (see below). Later, in 1898 he also discovered, by fractional distillation of liquid air, neon (“the new one”), krypton (“the hidden one”) and xenon (“the strange one”). The isolation of another element, fluorine, by Henri Moissan in Paris was honored with the 1906 Nobel Prize. In attempts to prepare artificial diamonds Moissan had also developed an electric furnace, and this was specifically mentioned in the prize citation, perhaps a reflection of the stipulation in Nobel’s will that the Prize for Chemistry can be given “for the most important discovery or improvement”.
Ernest Rutherford [Lord Rutherford since 1931], professor of physics in Manchester, was awarded the Nobel Prize for Chemistry in 1908 for his investigations of the chemistry of radioactive substances. The discovery of radioactivity had already been recognized with the Nobel Prize for Physics in 1903, but what Rutherford established was the transformation of one element into another, earlier the alchemist’s dream. In his studies of uranium disintegration he found two types of radiation, named a– and b-rays, and by their deviation in electric and magnetic fields he could show that a-rays consist of positively charged particles. His demonstration that these particles are helium nuclei came in the same year as he received the Nobel Prize. Even if the importance of Rutherford’s work for chemistry is obvious, he naturally had also received many nominations for the Nobel Prize for Physics (see Section 1).
In 1897 Eduard Buchner, at the time professor in Tübingen, published results demonstrating that the fermentation of sugar to alcohol and carbon dioxide can take place in the absence of yeast cells. Earlier it had generally been considered that living cells possess a “vital force”, which makes the life processes possible, even if a few prominent chemists, foremost Jöns Jacob Berzelius and Justus von Liebig, had advocated a chemical basis for life. The vitalistic outlook had been fiercely defended by Louis Pasteur, who maintained that alcoholic fermentation can only occur in the presence of living yeast cells. Buchner’s experiments showed unequivocally that fermentation is a catalytic process caused by the action of enzymes, as had been suggested by Berzelius for all life processes, and Buchner called his extract zymase (“enzymes in yeast”). Because of Buchner’s experiment, 1897 is generally regarded as the birth date for biochemistry proper. Buchner was awarded the Nobel Prize for Chemistry in 1907, when he was professor at the agricultural college in Berlin. This confirmed the prediction of his former teacher, Adolf von Baeyer: “This will make him famous, in spite of the fact that he lacks talent as a chemist.”
3. The Nobel Prizes for Chemistry 1911-2000
A survey of the Nobel Prizes for Chemistry awarded during the 20th century, reveals that the development of this field includes breakthroughs in all of its branches, with a certain dominance for progress in physical chemistry and its subcategories (chemical thermodynamics and chemical change), in chemical structure, in several areas of organic chemistry as well as in biochemistry. Of course, the borders between different areas are diffuse, therefore many Laureates will be mentioned in more than one place.
3.1 General and physical chemistry
The Nobel Prize for Chemistry in 1914 was awarded to Theodore William Richards of Harvard University for “his accurate determinations of the atomic weight of a large number of chemical elements”. Most atomic weights in Cannizzaros table (see Section 1.2) had already been determined in the 19th century, particularly by the Belgian chemist Jean Servais Stas, but Richards showed that many of them were in error, mainly because Stas had worked with very concentrated solutions, leading to co-precipitation. In 1913 Richards had discovered that the atomic weight of natural lead and of that formed in radioactive decay of uranium minerals differ. This pointed to the existence of isotopes, i.e. atoms of the same element with different atomic weights, which was accurately demonstrated by Francis William Aston at Cambridge University, with the aid of an instrument developed by him, the mass spectrograph. Aston also showed that the atomic weights of pure isotopes are, within the resolution of his experiment, integral numbers, with the exception of hydrogen, for which he obtained the atomic weight 1.008. For his achievements Aston received the Nobel Prize for Chemistry in 1922.
One branch of physical chemistry deals with chemical events at the interface of two phases, for example, solid and liquid, and phenomena at such interfaces have important applications all the way from technical to physiological processes. Detailed studies of adsorption on surfaces, were carried out by Irving Langmuir at the research laboratory of General Electric Company, and when he was awarded the Nobel Prize for Chemistry in 1932, he was the first industrial scientist to receive this distinction.
Two of the Prizes for Chemistry in more recent decades have been given for fundamental work in the application of spectroscopic methods to chemical problems. Spectroscopy had already been recognized with Prizes for Physics in 1952, 1955 and 1961, when Gerhard Herzberg, a physicist at the University of Saskatchewan, received the Nobel Prize for Chemistry in 1971 for his molecular spectroscopy studies “of the electronic structure and geometry of molecules, particularly free radicals”. The most used spectroscopic method in chemistry is undoubtedly NMR (nuclear magnetic resonance), and Richard R. Ernst at ETH in Zürich was given the Nobel Prize for Chemistry in 1991 for “the development of the methodology of high resolution nuclear magnetic resonance (NMR) spectroscopy”. Ernst’s methodology has now made it possible to determine the structure in solution (in contrast to crystals; cf. Section 3.5) of large molecules, such as proteins.
3.2 Chemical thermodynamics
The first Nobel Prize for Chemistry, that to van’t Hoff, was in part for work in chemical thermodynamics, and many later contributions in this area have also been recognized with Nobel Prizes. Already in 1920 Walther Hermann Nernst of Berlin received this award for work in thermochemistry, despite a 16-year opposition to this recognition from Arrhenius [2]. Nernst had shown that it is possible to determine the equilibrium constant for a chemical reaction from thermal data, and in so doing he formulated what he himself called the third law of thermodynamics. This states that the entropy, a thermodynamic quantity, which is a measure of the disorder in the system, approaches zero as the temperature goes towards absolute zero. van’t Hoff had derived the mass action equation in 1886, with the aid of the second law which says, that the entropy increases in all spontaneous processes [this had already been done in 1876 by J. Willard Gibbs at Yale, who certainly had deserved a Nobel Prize, but his work had been published in an obscure place]. According to the second law, heat of reaction is not an accurate measure of chemical equilibrium, as had been assumed by earlier investigators. But Nernst showed in 1906 that it is possible with the aid of the third law, to derive the necessary parameters from the temperature dependence of thermochemical quantities.
To prove his heat theorem (the third law) Nernst carried out thermochemical measurements at very low temperatures, and such studies were extended in the 1920s by G.N. Lewis (see Section 1.1) in Berkeley. Lewis’s new formulation of the third law was confirmed by his student William Francis Giauque, who extended the temperature range experimentally accessible by introducing the method of adiabatic demagnetization in 1933. With this he managed to reach temperatures a few thousandths of a degree above absolute zero and could thereby provide extremely accurate entropy estimates. He also showed that it is possible to determine entropies from spectroscopic data. Giauque was awarded the Nobel Prize for Chemistry in 1949 for his contributions to chemical thermodynamics.
The next Nobel Prize given for work in thermodynamics went to Lars Onsager of Yale University in 1968 for contributions to the thermodynamics of irreversible processes. Classical thermodynamics deals with systems at equilibrium, in which the chemical reactions are said to be reversible, but many chemical systems, for example, the most complex of all, living organisms, are far from equilibrium and their reactions are said to be irreversible. With the aid of statistical mechanics Onsager developed in 1931 his so-called reciprocal relations, describing the flow of matter and energy in such systems, but the importance of his work was not recognized until the end of the 1940s. A further step forward in the development of non-equilibrium thermodynamics was taken by Ilya Prigogine in Bruxelles, whose theory of dissipative structures was awarded the Nobel Prize for Chemistry in 1977.
3.3 Chemical change
The chief method to get information about the mechanism of chemical reactions is chemical kinetics, i.e. measurements of the rate of the reaction as a function of reactant concentrations as well as its dependence on temperature, pressure and reaction medium. Important work in this area had been done already in the 1880s by two of the early Laureates, van’t Hoff and Arrhenius, who showed that it is not enough for molecules to collide for a reaction to take place. Only molecules with sufficient kinetic energy in the collision do, in fact, react, and Arrhenius derived an equation in 1889 allowing the calculation of this activation energy from the temperature dependence of the reaction rate. With the advent of quantum mechanics in the 1920s (see Section 3.4), Eyring developed his transition-state theory in 1935 and this showed that the activation entropy is also important. Strangely, Eyring never received a Nobel Prize (see Section 1.2).
In 1956 Sir Cyril Norman Hinshelwood of Oxford and Nikolay Nikolaevich Semenov from Moscow shared the Nobel Prize for Chemistry “for their researches into the mechanism of chemical reactions”. Among Hinshelwood’s major contributions his detailed elucidation of the mechanism for the reaction between oxygen and hydrogen can be mentioned, whereas Semenov’s award was for his studies of so-called chain reactions.
A limit in investigating reaction rates is set by the speed with which the reaction can be initiated. If this is done by rapid mixing of the reactants, the time limit is about one thousandth of a second (millisecond). In the 1950s Manfred Eigen from Göttingen developed chemical relaxation methods that allow measurements in times as short as a thousandth or a millionth of a millisecond (microseconds or nanoseconds). The methods involve disturbing an equilibrium by rapid changes in temperature or pressure and then follow the passage to a new equilibrium. Another way to initiate some reactions rapidly is flash photolysis, i.e. by short light flashes, a method developed by Ronald G.W. Norrish at Cambridge and George Porter (Lord Porter since 1990) in London. Eigen received one-half and Norrish and Porter shared the other half of the Nobel Prize for Chemistry in 1967. The milli- to picosecond time scales gave important information on chemical reactions. However, it was not until it was possible to generate femtosecond laser pulses (10-15 s) that it became possible to reveal when chemical bonds are broken and formed. Ahmed Zewail (born 1946 in Egypt) at California Institute of Technology received the Nobel Prize for Chemistry in 1999 for his development of “femtochemistry” and in particular for being the first to experimentally demonstrate a transition state during a chemical reaction. His experiments relate back to 1889 when Arrhenius (Nobel Prize, 1903) made the important prediction that there must exist intermediates (transition states) in the transformation from reactants to products. Henry Taube of Stanford University was awarded the Nobel Prize for Chemistry in 1983 “for his work on the mechanism of electron transfer reactions, especially in metal complexes”. Even if Taube’s work was on inorganic reactions, electron transfer is important in many catalytic processes used in industry and also in biological systems, for example, in respiration and photosynthesis. The latest prize for work in chemical kinetics was that to Dudley R. Herschbach at Harvard University, Yuan T. Lee of Berkeley and John C. Polanyi from Toronto in 1986. Herschbach and his student Lee introduced the use of fluxes of molecules with well-defined direction and energy, molecular beams. By crossing two such beams they could study details of the reaction between molecules at extremely short times. Another important method to investigate such reaction details is infrared chemiluminescence, introduced by Polanyi. The emission of infrared radiation from the reaction products gives information on the energy distribution in the molecules.
3.4 Theoretical chemistry and chemical bonding
Quantum mechanics, developed in the 1920s, offered a tool towards a more basic understanding of chemical bonds. In 1927 Walter Heitler and Fritz London showed that it is possible to solve exactly the relevant equations for the hydrogen molecule ion, i.e. two hydrogen nuclei sharing a single electron, and thereby calculate the attractive force between the nuclei. For molecules containing more than three elementary particles, even the hydrogen molecule with Lewis’s two-electron bond (see Section 1.1), the equation can, however, not be solved exactly, so one has to resort to approximate methods. A pioneer in developing such methods was Linus Pauling at California Institute of Technology, who was awarded the Nobel Prize for Chemistry in 1954 “for his research into the nature of the chemical bond …” Pauling’s valence-bond (VB) method is rigorously described in his 1935 book Introduction to Quantum Mechanics (written together with E. Bright Wilson, Jr., at Harvard). A few years later (1939) he published an extensive non-mathematical treatment in The Nature of the Chemical Bond, a book which is one of the most read and influential in the entire history of chemistry. Pauling was not only a theoretician, but he also carried out extensive investigations of chemical structure by X-ray diffraction (see Section 3.5). On the basis of results with small peptides, which are building blocks of proteins, he suggested the a-helix as an important structural element. Pauling was awarded the Nobel Peace Prize for 1962, and he is the only person to date to have won two unshared Nobel Prizes.
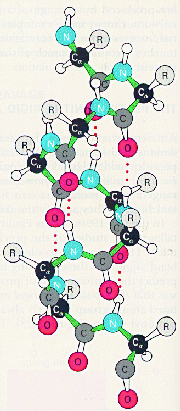
Pauling’s ![]() -helix
-helix
![]() -carbon atoms are black, other carbon atoms grey, nitrogen atoms blue, oxygen atoms red and hydrogen atoms white; R designates amino-acid side chains. The dotted red lines are hydrogen bonds between amide and carbonyl groups in the peptide bonds.
-carbon atoms are black, other carbon atoms grey, nitrogen atoms blue, oxygen atoms red and hydrogen atoms white; R designates amino-acid side chains. The dotted red lines are hydrogen bonds between amide and carbonyl groups in the peptide bonds.
Pauling’s VB method cannot give an adequate description of chemical bonding in many complicated molecules, and a more comprehensive treatment, the molecular-orbital (MO) method, was introduced already in 1927 by Robert S. Mulliken from Chicago and later developed further by him as well as by many other investigators. MO theory considers, in quantum-mechanical terms, the interaction between all atomic nuclei and electrons in a molecule. Mulliken also showed that a combination of MO calculations with experimental (spectroscopic) results provides a powerful tool for describing bonding in large molecules. Mulliken received the Nobel Prize for Chemistry in 1966.
Theoretical chemistry has also contributed significantly to our understanding of chemical reaction mechanisms. In 1981 the Nobel Prize for Chemistry was shared between Kenichi Fukui in Kyoto and Roald Hoffmann of Cornell University “for their theories, developed independently, concerning the course of chemical reactions”. Fukui introduced in 1952 the frontier-orbital theory, according to which the occupied MO with the highest energy and the unoccupied one with the lowest energy have a dominant influence on the reactivity of a molecule. Hoffmann formulated in 1965, together with Robert B. Woodward (see Section 3.8), rules based on the conservation of orbital symmetry, for the reactivity and stereochemistry in chemical reactions.
Rudolph A. Marcus published during ten years, starting in 1956, a series of seminal papers on a comprehensive theory for the rates electron-transfer reactions, the experimental study of which had given Taube a Nobel Prize in 1983 (see Section 3.3). Marcus’s theory predicts how the rate varies with the driving force for the reaction, i.e. the difference in energy between reactants and products, and counter to intuition he found that it does not increase continuously, but goes through a maximum, into the Marcus inverted region, which has later been confirmed experimentally. Marcus was awarded the Nobel Prize for Chemistry in 1992.
The latest Nobel Prize for work in theoretical chemistry was given in 1998 to Walter Kohn of Santa Barbara and John A. Pople of Northwestern University (but a British citizen). The prize to Kohn, a theoretical physicist, was based on his development of density-functional theory, which facilitates detailed calculations both of the geometrical structures of complex molecules and of the energy map of chemical reactions. Pople, a mathematician (but now Professor of Chemistry), was awarded “for his development of computational methods in quantum chemistry”. In particular, Pople has designed computer programs based on classical quantum theory as well as on density-functional theory.
3.5 Chemical structure
The most commonly used method to determine the structure of molecules in three dimensions is X-ray crystallography. The diffraction of X-rays was discovered by Max von Laue in 1912, and this gave him the Nobel Prize for Physics in 1914. Its use for the determination of crystal structure was developed by Sir William Bragg and his son, Sir Lawrence Bragg, and they shared the Nobel Prize for Physics in 1915. The first Nobel Prize for Chemistry for the use of X-ray diffraction went to Petrus (Peter) Debye, then of Berlin, in 1936. Debye did not study crystals, however, but gases, which give less distinct diffraction patterns. He also employed electron diffraction and the measurement of dipole moments to get structural information. Dipole moments are found in molecules, in which the positive and negative charge is unevenly distributed (polar molecules).
Many Nobel Prizes have been awarded for the determination of the structure of biological macromolecules (proteins and nucleic acids). Proteins are long chains of amino-acids, as shown by Emil Fischer (see Section 2), and the first step in the determination of their structure is to determine the order (sequence) of these building blocks. An ingenious method for this tedious task was developed by Frederick Sanger of Cambridge, and he reported the amino-acid sequence for a protein, insulin, in 1955. For this achievement he was awarded the Nobel Prize for Chemistry in 1958. Sanger later received part of a second Nobel Prize for Chemistry for a method to determine the nucleotide sequence in nucleic acids (see Section 3.12), and he is the only scientist so far who has won two Nobel Prizes for Chemistry.
The first protein crystal structures were reported by Max Perutz and Sir John Kendrew in 1960, and these two investigators shared the Nobel Prize for Chemistry in 1962. Perutz had started studying the oxygen-carrying blood pigment, hemoglobin, with Sir Lawrence Bragg in Cambridge already in 1937, and ten years later he was joined by Kendrew, who looked at crystals of the related muscle pigment, myoglobin. These proteins are both rich in Pauling’s a-helix (see Section 3.4), and this made it possible to discern the main features of the structures at the relatively low resolution first used. The same year that Perutz and Kendrew won their prize, the Nobel Prize for Physiology or Medicine went to Francis Crick, James Watson and Maurice Wilkins “for their discoveries concerning the molecular structure of nucleic acids … .” Two years later (1964) Dorothy Crowfoot Hodgkin received the Nobel Prize for Chemistry for determining the crystal structures of penicillin and vitamin B12.
Two later Nobel Prizes for Chemistry in the crystallographic field were given for work on structures of relatively small molecules. William N. Lipscomb of Harvard received the prize in 1976 “for his studies on the structures of boranes illuminating problems of chemical bonding”. In 1985 Herbert A. Hauptman of Buffalo and Jerome Karle of Washington, DC, shared the prize for “the development of direct methods for the determination of crystal structures”. Their methods are called direct, because they yield the structure directly from the diffraction data collected, and they have been indispensable in the determination of the structures of a large number of natural products.
Crystallographic electron microscopy was developed by Sir Aaron Klug in Cambridge, who was awarded the Nobel Prize for Chemistry in 1982. With this technique Klug has investigated the structure of large nucleic acid-protein complexes, such as viruses and chromatin, the carrier of the genes in the cell nucleus. Many of the most important life processes are carried out by proteins associated with biological membranes. This is, for example, true of the two key processes in energy metabolism, respiration and photosynthesis. Attempts to prepare crystals of membrane proteins for structural studies were, however, for many years unsuccessful, but in 1982 Hartmut Michel, then at the Max-Planck-Institut in Martinsried, managed to crystallize a photosynthetic reaction center after a painstaking series of experiments. He then proceeded to determine the three-dimensional structure of this protein complex in collaboration with Johann Deisenhofer and Robert Huber, and this was published in 1985. Deisenhofer, Huber and Michel shared the Nobel Prize for Chemistry in 1988. Michel has later also crystallized and determined the structure of the terminal enzyme in respiration, and his two structures have allowed detailed studies of electron transfer (cf. Sections 3.3 and 3.4) and its coupling to proton pumping, key features of the chemiosmotic mechanism for which Peter Mitchell had already received the Nobel Prize for Chemistry in 1978 (see Section 3.12). Functional and structural studies on the enzyme ATP synthase, connected to this proton pumping mechanism, was awarded one-half of the Nobel Prize for Chemistry in 1997, shared between Paul D. Boyer and John Walker (see Section 3.12).
3.6 Inorganic and nuclear chemistry
Much of the progress in inorganic chemistry during the 20th century has been associated with investigations of coordination compounds, i.e., a central metal ion surrounded by a number of coordinating groups, called ligands. In 1893 Alfred Werner in Zürich presented his coordination theory, and in 1905 he summarized his investigations in this new field in a book (Neuere Anschauungen auf dem Gebiete der anorganischen Chemie), which appeared in no less than five editions from 1905-1923. Compounds in which a metal ion binds several other molecules (ligands), for example, ammonia, had earlier been thought to have a linear structure, in accord with a theory advanced by the Swedish chemist Wilhelm Blomstrand in Lund. Werner showed that such a structure is inconsistent with some experimental facts, and he suggested instead that all the ligand molecules are bound directly to the metal ion. Werner was awarded the Nobel Prize for Chemistry in 1913. Taube’s investigations of electron transfer, awarded in 1983 (see Section 3.3), were mainly carried out with coordination compounds, and vitamin B12 as well as the proteins hemoglobin and myoglobin, investigated by the Laureates Hodgkin, Perutz and Kendrew (see Section 3.5), also belong to this category.
Another early prize for work in inorganic chemistry was that to Fritz Haber from Berlin in 1918 “for the synthesis of ammonia from its elements”, i.e., from nitrogen and hydrogen. The importance of this synthesis is above all in its industrial application in the form of the Haber-Bosch method, which had been developed by Carl Bosch as an improvement (cf. Nobel’s will) of Haber’s original procedure. It allows the manufacture of ammonia on a large scale, and the ammonia can then be used for the production of many different nitrogen-containing chemicals. Bosch shared the Nobel Prize for Chemistry with Friedrich Bergius in 1931 (see Section 3.13).
Much inorganic chemistry in the early 1900s was a consequence of the discovery of radioactivity in 1896, for which Henri Becquerel from Paris was awarded the Nobel Prize for Physics in 1903, together with Pierre and Marie Curie. In 1911 Marie Curie received the Nobel Prize for Chemistry for her discovery of the elements radium and polonium and for the isolation of radium and studies of its compounds, and this made her the first investigator to be awarded two Nobel Prizes. The prize in 1921 went to Frederick Soddy of Oxford for his work on the chemistry of radioactive substances and on the origin of isotopes. In 1934 Frédéric Joliot and his wife Irène Joliot-Curie, the daughter of the Curies, discovered artificial radioactivity, i.e., new radioactive elements produced by the bombardment of non-radioactive elements with a-particles or neutrons. They were awarded the Nobel Prize for Chemistry in 1935 for “their synthesis of new radioactive elements”.
Many elements are mixtures of non-radioactive isotopes (see Section 3.1), and in 1934 Harold Urey of Columbia University had been given the Nobel Prize for Chemistry for his isolation of heavy hydrogen (deuterium). Urey had also separated uranium isotopes, and his work was an important basis for the investigations by Otto Hahn from Berlin. In attempts to make transuranium elements, i.e., elements with a higher atomic number than 92 (uranium), by radiating uranium atoms with neutrons, Hahn discovered that one of the products was barium, a lighter element. Lise Meitner, at the time a refugee from Nazism in Sweden, who had earlier worked with Hahn and taken the initiative for the uranium bombardment experiments, provided the explanation, namely, that the uranium atom was cleaved and that barium was one of the products [3]. Hahn was awarded the Nobel Prize for Chemistry in 1944 “for his discovery of the fission of heavy nuclei”, and it can be wondered why Meitner was not included. Hahn’s original intention with his experiments was later achieved by Edwin M. McMillan and Glenn T. Seaborg of Berkeley, who were given the Nobel Prize for Chemistry in 1951 for “discoveries in the chemistry of transuranium elements”.
The use of stable as well as radioactive isotopes have important applications, not only in chemistry, but also in fields as far apart as biology, geology and archeology. In 1943 George de Hevesy from Stockholm received the Nobel Prize for Chemistry for his work on the use of isotopes as tracers, involving studies in inorganic chemistry and geochemistry as well as on the metabolism in living organisms. The prize in 1960 was given to Willard F. Libby of the University of California, Los Angeles (UCLA), for his method to determine the age of various objects (of geological or archeological origin) by measurements of the radioactive isotope carbon-14.
3.7 General organic chemistry
Contributions in organic chemistry have led to more Nobel Prizes for Chemistry than work in any other of the traditional branches of chemistry. Like the first prize in this area, that to Emil Fischer in 1902 (see Section 2), most of them have, however, been awarded for advances in the chemistry of natural products and will be treated separately (Section 3.9). Another large group, preparative organic chemistry, has also been given its own section (Section 3.8), and here only the prizes for more general contributions to organic chemistry will be discussed. In 1969 the Nobel Prize for Chemistry went to Sir Derek H. R. Barton from London, and Odd Hassel from Oslo for developing the concept of conformation, i.e. the spatial arrangement of atoms in molecules, which differ only by the orientation of chemical groups by rotation around a single bond. This stereochemical concept rests on the original suggestion by van’t Hoff of the tetrahedral arrangement of the four valences of the carbon atom (see Section 2), and most organic molecules exist in two or more stable conformations.
The Nobel Prize for Chemistry in 1975 to Sir John Warcup Cornforth of the University of Sussex and Vladimir Prelog of ETH in Zürich was also based on research in stereochemistry. Not only can a compound have more than one geometric form, but chemical reactions can also have specificity in their stereochemistry, thereby forming a product with a particular three-dimensional arrangement of the atoms. This is especially true of reactions in living organisms, and Cornforth has mainly studied enzyme-catalyzed reactions, so his work borders onto biochemistry (Section 3.12). One of Prelog’s main contributions concerns chiral molecules, i.e. molecules that have two forms differing from one another as the right hand does from the left. Stereochemically specific reactions have great practical importance, as many drugs, for example, are active only in one particular geometric form.
Organometallic compounds constitute a group of organic molecules containing one or more carbon-metal bond, and they are thus the organic counterpart to Werner’s inorganic coordination compounds (see Section 3.6). In 1952 Ernst Otto Fischer and Sir Geoffrey Wilkinson independently described a completely new group of organometallic molecules, called sandwich compounds (see figure below). In such compounds a metal ion is bound not to a single carbon atom but is “sandwiched” between two aromatic organic molecules. Fischer and Wilkinson shared the Nobel Prize for Chemistry in 1973.
 |
| Sandwich compounds |
Work on the interaction of metal ions with organic molecules was also recognized by the prize in 1987, which was shared by Donald J. Cram of UCLA, Jean-Marie Lehn from Strasbourg (and Paris) and Charles J. Pedersen of the Du Pont Company. These three investigators have synthesized molecules with a ring structure, in which the hole in their middle specifically recognizes and binds different metal ions. They can, for example, distinguish between closely related ions, such as those of sodium and potassium, and thus they mimic enzymes in their specificity. The first such compound was synthesized by Pedersen in 1967, and later Lehn and Cram developed increasingly sophisticated organic compounds with cavities and cages in which not only metal ions but other molecules are bound. This research has applications in the whole spectrum of the chemical field, from inorganic chemistry to biochemistry.
George A. Olah from the University of Southern California was awarded the Nobel Prize for Chemistry in 1994 “for his contributions to carbocation chemistry”. Already in the 1920s and 1930s chemists had suggested that positively charged ions of hydrocarbons are formed as short-lived intermediates in organic chemical reactions. Such carbocations were, however, thought to be so reactive and unstable that it would be impossible to prepare them in quantity. Olah’s investigations, starting in the 1960s, contradicted this supposition, since he showed that stable carbocations can be prepared by the use of a new type of extremely acidic compounds (“superacids”), and carbocation chemistry now has a prominent position in all modern textbooks of organic chemistry.
The preparation of a new form of carbon compounds was also recognized by the Nobel Prize for Chemistry in 1996 to Robert F. Curl, Jr., of Rice University, Sir Harold W. Kroto of the University of Sussex and Richard E. Smalley of Rice University. These investigators had in 1985 discovered compounds, called fullerenes, in which 60 or 70 carbon atoms are bound together in clusters in the form of a ball (see figure below). The designation fullerenes is taken from the name of an American architect, R. Buckminster Fuller, who had designed a dome having the form of a football for the 1967 Montreal World Exhibition.
Fullerenes
3.8 Preparative organic chemistry
One of the chief goals of the organic chemist is to be able to synthesize increasingly complex compounds of carbon in combination with various other elements, such as hydrogen, oxygen, nitrogen, sulfur and phosphorus. The first Nobel Prize for Chemistry recognizing pioneering work in preparative organic chemistry was that to Victor Grignard from Nancy and Paul Sabatier from Toulouse in 1912. Grignard had discovered that organic halides can form compounds with magnesium. These compounds, now generally called Grignard reagents, are very reactive, and they are consequently widely used for synthetic purposes. Sabatier was given the prize for developing a method to hydrogenate organic compounds in the presence of metallic catalysts. With his method oils can be converted to saturated fats, and it is, for example, used for margarine production and other industrial processes.
The prize in 1950 was presented to Otto Diels from Kiel and Kurt Alder from Cologne “for their discovery and development of the diene synthesis”, also called the Diels-Alder reaction. In this reaction, which was developed already in 1928, organic compounds containing two double bonds (“dienes”) can effect the syntheses of many cyclic organic substances. During the decades following the original work several industrial applications of the Diels-Alder reaction have been found, for example, in the production of plastics, which may explain the lateness of the prize.
The German organic chemist Hans Fischer from Munich had already done significant work on the structure of hemin, the organic pigment in hemoglobin, when he synthesized it from simpler organic molecules in 1928. He also contributed much to the elucidation of the structure of chlorophyll, and for these important achievements he was awarded the Nobel Prize for Chemistry in 1930 (cf. Section 3.5). He finished his determination of the structure of chlorophyll in 1935, and by the time of his death he had almost completed its synthesis as well.
Robert Burns Woodward from Harvard is rightly considered the founder of the most advanced, modern art of organic synthesis. He designed methods for the total synthesis of a large number of complicated natural products, for example, cholesterol, chlorophyll and vitamin B12. He received the Nobel Prize for Chemistry in 1965, and he would probably have received a second chemistry prize in 1981 for his part in the formulation of the Woodward-Hoffmann rules (see Section 3.4), had it not been for his early death. Work in synthetic organic chemistry was also recognized in 1979 with the prize to Herbert C. Brown of Purdue University and Georg Wittig from Heidelberg, who had developed the use of boron- and phosphorus-containing compounds, respectively, into important reagents in organic synthesis. Another master in chemical synthesis is Elias James Corey from Harvard, who received the prize in 1990. He had made a brilliant analysis of the theory of organic synthesis, which permitted him to synthesize biologically active compounds of a complexity earlier considered impossible.
The Nobel Prize for Chemistry in 1984 was given to Robert Bruce Merrifield of Rockefeller University “for his development of methodology for chemical synthesis on a solid matrix”. Specifically, Merrifield applied this ingenious idea to the synthesis of large peptides and small proteins, for example, ribonuclease (cf. Section 3.12), but the principle has later also been applied to nucleic acid chemistry. In earlier methods each intermediate in the synthesis had to be isolated, which resulted in a drastic drop in yield in syntheses involving a large number of consecutive steps. In Merrifield’s method these isolation steps are replaced by a simple washing procedure, which removes by-products as well as remaining starting materials, and in this way substantial losses are avoided.
3.9 Chemistry of natural product
The synthesis of complex organic molecules must be based on detailed knowledge of their structure. Early work on plant pigments was carried out by Richard Willstätter, a student of Adolf von Baeyer from Munich (see Section 2). Willstätter showed a structural relatedness between chlorophyll and hemin, and he demonstrated that chlorophyll contains magnesium as an integral component. He also carried out pioneering investigations on other plant pigments, such as the carotenoids, and he was awarded the Nobel Prize for Chemistry in 1915 for these achievements. Willstätter’s work laid the ground for the synthetic accomplishments of Hans Fischer (see Section 3.8). In addition, Willstätter contributed to the understanding of enzyme reactions.
The prizes for 1927 and 1928 were both presented to Heinrich Otto Wieland from Munich and Adolf Windaus from Göttingen, respectively, at the Nobel ceremony in 1928. These two chemists had done closely related work on the structure of steroids. The award to Wieland was primarily for his investigations of bile acids, whereas Windaus was recognized mainly for his work on cholesterol and his demonstration of the steroid nature of vitamin D. Wieland had already in 1912, before his prize-winning work, formulated a theory for biological oxidation, according to which removal of hydrogen (dehydrogenation) rather than reaction with oxygen is the dominating process.
Investigations on vitamins were recognized in 1937 and 1938 with the prizes to Sir Norman Haworth from Birmingham and Paul Karrer from Zürich and to Richard Kuhn from Heidelberg. Haworth did outstanding work in carbohydrate chemistry, establishing the ring structure of glucose. He was the first chemist to synthesize vitamin C, and this is the basis for the present large-scale production of this nutrient. Haworth shared the prize with Karrer, who determined the structure of carotene and of vitamin A. Kuhn also worked on carotenoids, and he published the structure of vitamin B2 at the same time as Karrer. He also isolated vitamin B6. In 1939 the Nobel Prize for Chemistry was shared between Adolf Butenandt from Berlin and Leopold Ruzicka (1887-1976) of ETH, Zurich. Butenandt was recognized “for his work on sex hormones”, having isolated estrone, progesterone and androsterone. Ruzicka synthesized androsterone and also testosterone.
The awards for outstanding work in natural-product chemistry continued after World War II. In 1947 Sir Robert Robinson from Oxford received the prize for his studies on plant substances, particularly alkaloids, such as morphine. Robinson also synthesized steroid hormones, and he elucidated the structure of penicillin. Many hormones are of a polypeptide nature, and in 1955 Vincent du Vigneaud of Cornell University was given the prize for his synthesis of two such hormones, vasopressin and oxytocin. Finally, in this area, Alexander R. Todd (Lord Todd since 1962) was recognized in 1957 “for his work on nucleotides and nucleotide co-enzymes”. Todd had synthesized ATP (adenosine triphosphate) and ADP (adenosine diphosphate), the main energy carriers in living cells, and he determined the structure of vitamin B12 (cf. Section 3.5) and of FAD (flavin-adenine dinucleotide).
3.10 Analytical chemistry and separation science
Inorganic chemists, organic chemists and biochemists develop analytical methods as part of their regular research. It is consequently natural that not many Nobel Prizes have been awarded for contributions specifically in analytical chemistry. One such prize was, however, that to Fritz Pregl from Graz in 1923 for his development of organic microanalysis. The medical biochemist from Uppsala, Olof Hammarsten, who gave the presentation speech as Chairman of the Nobel Committee for Chemistry, stressed that Pregl’s work constituted an improvement rather than a discovery, in accord with Nobel’s will. Pregl modified existing methods for quantitative elemental analysis of organic substances to handle very small quantities, which saved time, labor and expense. Another prize in analytical chemistry was given to Jaroslav Heyrovsky from Prague in 1959 for his development of polarographic methods of analysis. In these a dropping mercury electrode is employed to determine current-voltage curves for electrolytes. A given ion reacts at a specific voltage, and the current is a measure of the concentration of this ion.
The analysis of macromolecular constituents in living organisms requires specialized methods of separation. One such method is ultracentrifugation, developed by The Svedberg from Uppsala a few years before he was awarded the Nobel Prize for Chemistry in 1926 “for his work on disperse systems” (see Section 3.11). Svedberg’s student, Arne Tiselius, studied the migration of protein molecules in an electric field, and with this method, named electrophoresis, he demonstrated the complex nature of blood proteins. Tiselius also refined adsorption analysis, a method first used by the Russian botanist, Michail Tswett, for the separation of plant pigments and named chromatography by him. In 1948 Tiselius was given the prize for these achievements. A few years later (1952) Archer J.P. Martin from London and Richard L.M. Synge from Bucksburn (Scotland) shared the prize “for their invention of partition chromatography”, and this method was a major tool in many biochemical investigations later awarded with Nobel Prizes (see Section 3.12).
3.11 Polymers and colloids
Polymeric substances in solution, including life constituents, such as proteins and polysaccharides, are in a colloidal state, i.e., they exist as suspensions of particles one-millionth to one-thousandth of a centimeter in size. In the case of the biological polymers the individual molecules are so large that they form a colloidal suspension, but many other substances can be obtained in a colloidal state. A much-studied example is aggregates of gold atoms, and the Nobel Prize for Chemistry for 1925 was given to Richard Zsigmondy from Göttingen for demonstrating the heterogeneous nature of such gold sols. He did this with the aid of an instrument, the ultramicroscope, which he had developed in collaboration with scientists at the Zeiss factory in Jena. With this instrument the particles and their motion can be observed by the light they scatter at a right angle to the direction of the illuminating light beam. Early work in colloid chemistry had also been carried out by Wolfgang Ostwald, son of the 1909 Laureate Wilhelm Ostwald, but this was not of a caliber earning him a Nobel Prize.
The Svedberg who received the Nobel Prize for Chemistry in 1926, also investigated gold sols. He used Zsigmond’s ultramicroscope to study the Brownian movement of colloidal particles, so named after the Scottish botanist Robert Brown, and confirmed a theory developed by Albert Einstein in 1905 and, independently, by M. Smoluchowski. His greatest achievement was, however, the construction of the ultracentrifuge, with which he studied not only the particle size distribution in gold sols but also determined the molecular weight of proteins, for example, hemoglobin. In the same year as Svedberg got the prize the Nobel Prize for Physics was awarded to Jean Baptiste Perrin of Sorbonne for developing equilibrium sedimentation in colloidal solutions, a method which Svedberg later perfected in his ultracentrifuge. Svedberg’s investigations with the ultracentrifuge and Tiselius’s electrophoresis studies (see Section 3.10) were instrumental in establishing that protein molecules have a unique size and structure, and this was a prerequisite for Sanger’s determination of their amino-acid sequence and the crystallographic work of Kendrew and Perutz (see Section 3.5).
In the 1920s Hermann Staudinger from Freiburg developed the concept of macromolecules. He synthesized many polymers, and he showed that they are long chain molecules. The large plastic industry is largely based on Staudinger’s work. In 1953 he received the Nobel Prize for Chemistry “for his discoveries in the field of macromolecular chemistry”. The prize in 1963 was shared by Karl Ziegler of the Max-Planck-Institute in Mülheim and Giulio Natta from Milan for their discoveries in polymer chemistry and technology. Ziegler demonstrated that certain organometallic compounds (see Section 3.7) can be used to effect polymerization reactions, and Natta showed that Ziegler catalysts can produce polymers with a highly regular three-dimensional structure. Another Nobel Prize for contributions in polymer chemistry was given to Paul J. Flory of Stanford in 1974. Flory carried out fundamental theoretical as well as experimental investigations of the physical chemistry of macromolecules, but his work also led to such important polymers as nylon and synthetic rubber. In 1977 a paper entitled “Synthesis of electrically conducting organic polymers: Halogen derivates of polyacetylene” was published in the Journal of the American Chemical Society, Chemical Communications. The authors of this paper, Alan J. Heeger of the University of California at Santa Barbara, Alan G. MacDiarmid of the University of Pennsylvania and Hideki Shirakawa of the University of Tsukuba, Japan were awarded the Nobel Prize for Chemistry in 2000 for this discovery. The conducting polymers have already given rise to a number of applications such as photodiodes and light-emitting diodes and have future potential to generate microelectronics based upon plastic materials.
3.12 Biochemistry
The second Nobel Prize for discoveries in biochemistry came in 1929, when Sir Arthur Harden from London and Hans von Euler-Chelpin from Stockholm shared the prize for investigations of sugar fermentation, which formed a direct continuation of Buchner’s work awarded in 1907. With his young co-worker, William John Young, Harden had shown in 1906 that fermentation requires a dialysable substance, called co-zymase, which is not destroyed by heat. Harden and Young also demonstrated that the process stops before all sugar (glucose) has been used up, but it starts again on addition of inorganic phosphate, and they suggested that hexose phosphates are formed in the early steps of fermentation. von Euler had done important work on the structure of co-zymase, shown to be nicotinamide adenine dinucleotide (NAD, earlier called DPN). As the number of Laureates can be three, it may seem appropriate for Young to have been included in the award, but Euler’s discovery was published together with Karl Myrbäck, and the number of Laureates is limited to three.
The next biochemical Nobel Prize was given in 1946 for work in the protein field. James B. Sumner of Cornell University received half the prize “for his discovery that enzymes can be crystallized” and John H. Northrop together with Wendell M. Stanley, both of the Rockefeller Institute, shared the other half “for their preparation of enzymes and virus proteins in a pure form”. Sumner had in 1926 crystalized an enzyme, urease, from jack beans and suggested that the crystals were the pure protein. His claim was, however, greeted with great scepticism, and the crystals were suggested to be inorganic salts with the enzyme adsorbed or occluded. Just a few years after Sumner’s discovery Northrop, however, managed to crystalize three digestive enzymes, pepsin, trypsin and chymotrypsin, and by painstaking experiments shown them to be pure proteins. Stanley started his attempt to purify virus proteins in the 1930s, but not until 1945 did he get virus crystals, and this then made it possible to show that viruses are complexes of protein and nucleic acid. The pioneering studies of these three investigators form the basis for the enormous number of new crystal structures of biological macromolecules, which have been published in the second half of the 20th century (cf. Section 3.5).
Several Nobel Prizes for Chemistry have been awarded for work in photosynthesis and respiration, the two main processes in the energy metabolism of living organisms (cf. Section 3.5). In 1961 Melvin Calvin of Berkeley received the prize for elucidating the carbon dioxide assimilation in plants. With the aid of carbon-14 (cf. Section 3.6) Calvin had shown that carbon dioxide is fixed in a cyclic process involving several enzymes. Peter Mitchell of the Glynn Research Laboratories in England was awarded in 1978 for his formulation of the chemiosmotic theory. According to this theory, electron transfer (cf. Sections 3.3 and 3.4) in the membrane-bound enzyme complexes in both respiration and photosynthesis, is coupled to proton translocation across the membranes, and the electrochemical gradient thus created is used to drive the synthesis of ATP (adenosine triphosphate), the energy storage molecule in all living cells. Paul D. Boyer of UCLA and John C. Walker of the MRC Laboratory in Cambridge shared one-half of the 1997 prize for their elucidation of the mechanism of ATP synthesis; the other half of the prize went to Jens C. Skou in Aarhus for the first discovery of an ion-transporting enzyme. Walker had determined the crystal structure of ATP synthase, and this structure confirmed a mechanism earlier proposed by Boyer, mainly on the basis of isotopic studies.
Luis F. Leloir from Buenos Aires was awarded in 1970 “for the discovery of sugar nucleotides and their role in the biosynthesis of carbohydrates”. In particular, Leloir had elucidated the biosynthesis of glycogen, the chief sugar reserve in animals and many microorganisms. Two years later the prize went with one half to Christian B. Anfinsen of NIH and the other half shared by Stanford Moore and William H. Stein, both from Rockefeller University, for fundamental work in protein chemistry. Anfinsen had shown, with the enzyme ribonuclease, that the information for a protein assuming a specific three-dimensional structure is inherent in its amino-acid sequence, and this discovery was the starting point for studies of the mechanism of protein folding, one of the major areas of present-day biochemical research. Moore and Stein had determined the amino-acid sequence of ribonuclease, but they received the prize for discovering anomalous properties of functional groups in the enzyme’s active site, which is a result of the protein fold.
Naturally a number of Nobel Prizes for Chemistry have been given for work in the nucleic acid field. In 1980 Paul Berg of Stanford received one half of the prize for studies of recombinant DNA, i.e. a molecule containing parts of DNA from different species, and the other half was shared by Walter Gilbert from Harvard and Frederick Sanger (see Section 3.5) for developing methods for the determination of the base sequences of nucleic acids. Berg’s work provides the basis of genetic engineering, which has led to the large biotechnology industry. Base sequence determinations are essential steps in recombinant-DNA technology, which is the rationale for Gilbert and Sanger sharing the prize with Berg. Sidney Altman of Yale and Thomas R. Cech of the University of Colorado shared the prize in 1989 “for their discovery of the catalytic properties of RNA”. The central dogma of molecular biology is: DNA –> RNA –> enzyme. The discovery that not only enzymes but also RNA possesses catalytic properties have led to new ideas about the origin of life. The 1993 prize was shared by Kary B. Mullis from La Jolla and Michael Smith from Vancouver, who both have given important contributions to DNA technology. Mullis developed the PCR (“polymerase chain reaction”) technique, which makes it possible to replicate millions of times a specific DNA segment in a complicated genetic material. Smith’s work forms the basis for site-directed mutagenesis, a technique by which it is possible to change a specific amino-acid in a protein and thereby illuminate its functional role.
3.13 Applied chemistry
A few Nobel Prizes for Chemistry have recognized contributions outside the conventional basic chemical fields. The prize in 1931 went to Carl Bosch and Friedrich Bergius , both from Heidelberg, “for the invention and development of chemical high pressure methods”. Bosch had modified Haber’s method for ammonia synthesis (see Section 3.6) to make it suitable for large-scale industrial use. Bergius used high-pressure methods to prepare oil by the hydrogenation of coal, and Bosch, like Bergius working at the large concern I. G. Farben, later improved the procedure by finding a good catalyst for the Bergius process.
Work in agricultural and nutritional chemistry led to the award of Artturi Ilmari Virtanen from Helsinki in 1945. The citation particularly stressed his development of the AIV method, so named after the inventor’s initials. Virtanen had first carried out biochemical studies of nitrogen fixation by plants with the aim of producing protein-rich crops. He then found that the fodder could be preserved with the aid of a mixture of sulfuric and nitric acid (AIV acid).
Finally, basic work in atmospheric and environmental chemistry was recognized in 1995 with the prize to Paul Crutzen, from the Netherlands, working at Stockholm University and later at the Max-Planck-Institute in Mainz, Mario Molina of MIT and F. Sherwood Rowland of UC, Irvine. These three investigators have studied in detail the chemical processes leading to the formation and decomposition of ozone in the atmosphere. In particular, they have shown that the atmospheric ozone layer is very sensitive to emission chemicals produced by human activity, and these discoveries have led to international legislation.
4. Concluding remarks
The first hundred years of Nobel Prizes for Chemistry give a beautiful picture of the development of modern chemistry. The prizes cover the whole spectrum of the basic chemical sciences, from theoretical chemistry to biochemistry, and also a number of contributions to applied chemistry. From a quantitative point of view, organic chemistry dominates with no less than 25 awards. This is not surprising, since the special valence properties of carbon result in an almost infinite variation in the structure of organic compounds. Also, a large number of the prizes in organic chemistry were given for investigations of the chemistry of natural products of increasing complexity and thus are on the border to biochemistry.
As many as 11 prizes have been awarded for biochemical discoveries. Even if the first biochemical prize was already given in 1907 (Buchner), only three awards in this area came in the first half of the century, illustrating the explosive growth of biochemistry in recent decades (8 prizes in 1970-1997). At the other end of the chemical spectrum, physical chemistry, including chemical thermodynamics and kinetics, dominates with 14 prizes, but there has also been 6 prizes in theoretical chemistry. Chemical structure is another large area with 8 prizes, including awards for methodological developments as well as for the determination of the structure of large biological molecules or molecular complexes. Industrial chemistry was first recognized in 1931 (Bergius, Bosch), but many more recent prizes for basic contributions lie close to industrial applications, for example, those in polymer chemistry.
Science is a truly international undertaking, but the western dominance of the Nobel scene is striking. No less than 49 scientists in the United States have received the Nobel Prize for Chemistry, but the majority have been given the prize after World War II. The first US prize was awarded in 1915 (for 1914, Richards), and only two more Americans got the prize before 1946 (Langmuir in 1932, Urey in 1934). German chemists form the second most awarded group with 26 Laureates, but 14 of these received the prize before 1945. Of the 25 British investigators recognized, on the other hand, no less than 19 got the prize in the second half of the century. France has 7 Laureates in chemistry, Sweden and Switzerland 5 each, and the Netherlands and Canada 3. One prize winner each is found in the following countries: Argentina, Austria, Belgium, Czechoslovakia, Denmark, Finland, Italy, Norway and Russia.
Extrapolating the trend of the 20th century Nobel Prizes for Chemistry, it is expected that in the 21st century theoretical and computational chemistry will flourish with the aid of the expansion of computer technology. The study of biological systems may become more dominant and move from individual macromolecules to large interactive systems, for example, in chemical signaling and in neural function, including the brain. And it is to be hoped that the next century will witness a wider national distribution of Laureates.
5. References
Westgren, A., Nobel – The Man and His Prizes, ed. Odelberg, W. (Elsevier, New York, 1972), pp. 279-385.
Kormos Barkan, D., Walther Nernst and the Transition in Modern Physical Science, (Cambridge University Press, 1999).
Rife, P., Lise Meitner and the Dawn of the Nuclear Age, (Birkhäuser, 1999).
* This article was published as a chapter of the book: “The Nobel Prize: The First 100 Years”, Agneta Wallin Levinovitz and Nils Ringertz, eds., Imperial College Press and World Scientific Publishing Co. Pte. Ltd., 2001.
Bo G. Malmström (b. 1927, d. 2000) was Professor of Biochemistry at Göteborg University in 1963-1993. Apart from his doctorate from Uppsala University, he held honorary doctorates from Muhlenberg College in the US and from Utrecht University. He was Visiting Professor at University of Southern California, University of California, Berkeley, Utrecht University and California Institute of Technology, and he also carried out research at Universitá di Roma and Universitá di Firenze. He published over 200 papers, particularly in inorganic biochemistry and bioenergetics. His main interest in later years was the terminal enzyme of respiration, cytochrome oxidase, and its function as a proton pump. In 1996 he started investigating protein folding of redox-active metalloproteins, especially copper proteins. He was a member of the Nobel Committee for Chemistry in 1972-1988 and its chairman in 1977-1988.
Bertil Andersson (b. 1948) is professor in Biochemistry and President of Linköping University, Sweden (1999-2003). He was head of the Dept. of Biochemistry (1987-1995), Dean of the Faculty of Chemical Sciences and prodean of the Science Faculty, (1996-1999) at the University of Stockholm. He has been elected Executive Director of the European Science Foundation for 2004. His honorary appointments include: Member of the Board of the Nobel Foundation, 2000- ; Member (and chairman) of the Nobel Committee for Chemistry 1989-1997, (1997); Member of the Royal Swedish Academy of Sciences, since 1989. He has been President of the chemical section of the Royal Swedish Academy of Sciences, since 1998; Member of Academiae Europea, since 1990; The Finnish Science Society, since 1991; The Australian Academy of Sciences, since 1999; The European Molecular Biology Organization (EMBO), since 1990. He has received Honorary doctorates from Turku University, 2000 and Umeå University, 2002. He has been a Visiting Professor at Imperial College, London since 1990. Prof. Andersson has published a total of 275 papers in photosynthesis research, biological membranes, protein and membrane purification, and light stress.
Jean-Marie Lehn – Biographical

I was born on September 30 1939 in Rosheim, a small medieval city of Alsace in France. My father, Pierre Lehn, then a baker, was very interested in music, played the piano and the organ and became later, having given up the bakery, the organist of the city. My mother Marie kept the house and the shop. I was the eldest of four sons and helped out in the shop with my first brother. I grew up in Rosheim during the years of the second world war, went to primary school after the war and, at age eleven, I entered high school, the Collège Freppel, located in Obernai, a small city about five kilometers from Rosheim. During these years I began to play the piano and the organ, and with time music has become my major interest outside science. My high school studies from 1950 to 1957 were in classics, with Latin, Greek, German, and English languages, French literature and, during the last year, philosophy, on which I was especially keen. However, I also became interested in sciences, especially chemistry, so that I obtained the baccalauréat in Philosophy in July 1957 and in Experimental Sciences in September of the same year.
I envisaged to study philosophy at the University of Strasbourg, but being still undecided, I began with first year courses in physical, chemical and natural sciences (SPCN). During this year 1957/58, I was impressed by the coherent and rigorous structure of organic chemistry. I was particularly receptive to the experimental power of organic chemistry, which was able to convert at will, it seemed, complicated substances into one another following well defined rules and routes. I bought myself compounds and glassware and began performing laboratory practice experiments at my parents home. The seed was sown, so that when, the next year, I followed the stimulating lectures of a newly appointed young professor, Guy Ourisson, it became clear to me that I wanted to do research in organic chemistry.
After having obtained the degree of Licencié-ès-Sciences (Bachelor), I entered Ourisson’s laboratory in October of 1960, as a junior member of the Centre National de la Recherche Scientifique in order to work towards a Ph.D. degree. This was the first decisive stage of my training. My work was concerned with conformational and physico-chemical properties of triterpenes. Being in charge of our first NMR spectrometer, I was led to penetrate more deeply into the arcanes of this very powerful physical method; this was to be of much importance for later studies. My first scientific paper in 1961 reported an additivity rule for substituent induced shifts of proton NMR signals in steroid derivatives.
Having obtained my degree of Docteur ès Sciences (Ph.D.) in June of 1963, I spent a year in the laboratory of Robert Burns Woodward at Harvard University, where I took part in the immense enterprise of the total synthesis of Vitamin B12. This was the second decisive stage of my life as a researcher. I also followed a course in quantum mechanics and performed my first computations with Roald Hoffmann. I had the chance to witness in 1964 the initial stages of what was to become the Woodward-Hoffmann rules.
After my return to Strasbourg, I began to work in the area of physical organic chemistry, where I could combine the knowledge acquired in organic chemistry, in quantum theory and on physical methods. It was clear that, in order to be able to better analyze physical properties of molecules, a powerful means was to synthesize compounds that would be especially well suited for revealing a given property and its relationships to structure. This orientation characterized the years 1965- 1970 of my activities and of my young laboratory, newly established after my appointment in 1966 as maître de conférences (assistant professor) at the Chemistry Department of the University of Strasbourg. Our main research topics were concerned with NMR studies of conformational rate processes, nitrogen inversion, quadrupolar relaxation, molecular motions and liquid structure, as well as ab initio quantum chemical computations of inversion barriers, of electronic structures and later on, of stereoelectronic effects.
While pursuing these projects, my interest for the processes occurring in the nervous system (stemming diffusely from the first year courses in biology as well as from my earlier inclination towards philosophy), led me to wonder how a chemist might contribute to their study. The electrical phenomena in nerve cells depend on sodium and potassium ion distributions across membranes. A possible entry into the field was to try to affect the processes which allow ion transport and gradients to be established. I related this to the then very recent observations that natural antibiotics were able to make membranes permeable to cations. It thus appeared possible to devise chemical substances that would display similar properties. The search for such compounds led to the design of cation cryptates, on which work was started in October 1967. This area of research expanded rapidly, taking up eventually the major part of my group and developing into what I later on termed “supramolecular chemistry”. Organic, inorganic and biological aspects of this field were explored and investigations are continuing. In 1976 another line of research was started in the area of artificial photosynthesis and the storage and chemical conversion of solar energy; it was first concerned with the photoly is of water and later with the photoreduction of carbon dioxide.
I was promoted associate professor in early 1970 and full professor in October of the same year. I spent the two spring semesters of 1972 and 1974 as visiting professor at Harvard University giving lectures and directing a research project. This relationship extended on a loose basis to 1980. In 1979, I was elected to the chair of “Chimie des Interactions Moléculaires” at the Collège de France in Paris. I took over the chemistry laboratory of the Collège de France when Alain Horeau retired in 1980 and thereafter divided my time between the two laboratories in Strasbourg and in Paris, a situation continuing up to the present. New lines of research developed, in particular on combining the recognition, transport and catalytic properties displayed by supramolecular species with the features of organized phases, the long range goal being to design and realize “molecular devices”, molecular components that would eventually be able to perform signal and information processing at the molecular level. A major research effort is presently also devoted to supramolecular self-organisation, the design and properties of “programmed” supramolecular systems.
The scientific work, performed over twenty years with about 150 collaborators from over twenty countries, has been described in about 400 publications and review papers. Over the years I was visiting professor at other institutions, the E.T.H. in Zürich, the Universities of Cambridge, Barcelona, Frankfurt.
In 1965 I married Sylvie Lederer and we have two sons, David (born 1966) and Mathias (born 1969).
This autobiography/biography was written at the time of the award and later published in the book series Les Prix Nobel/ Nobel Lectures/The Nobel Prizes. The information is sometimes updated with an addendum submitted by the Laureate.
Addendum, January 2006
Research Activities – Update
Over the years, the studies in supramolecular chemistry in my laboratory at the Université Louis Pasteur in Strasbourg extended into a broad new area at the interface of chemistry with biology: self-organization processes, making use of molecular recognition to control and direct the spontaneous formation of functional architectures of high complexity.
Realizing that recognition implies information, led to the concepts of molecular programming and of programmed chemical systems, undergoing self-organization on the basis of the molecular storage of information and its processing at the supramolecular level through algorithms defined by the specific features of the intermolecular interaction patterns involved in the system considered.
These investigations provide steps towards a progressive understanding of the passage from condensed matter to organized matter, of which living organisms represent the highest expression. They seek to lay the chemical, molecular and supramolecular foundations on which the highly complex events of biological self-organization are built and to provide means for analyzing their mechanism as well as for acting on them.
A variety of chemical self-organizing systems of either organic or inorganic nature were designed and studied, leading to the generation of various organic and inorganic supramolecular architectures from molecular components assembled respectively through hydrogen-bonding and ligand-metal ion recognition processes, such as – the “helicates“, double, triple, and circular inorganic helices, – multicompartmental nanocylinders, –”grid-type” entities, ordered polymetallic arrays presenting a range of intriguing physico-chemical properties (multiple redox states, spin crossover magnetism, etc.).
The combination of molecular “softwares” specific for the assembly of different architectures into a single programme gave rise to the concept of multiple expression of molecular information, whereby the supramolecular processing of the information by different recognition algorithms generates different outputs, in a one code/several products mode.
Introducing the concepts and results of supramolecular chemistry into materials science, led to the emergence and the development of supramolecular polymer chemistry, as a new area in polymer chemistry.
On the other hand, the elaboration of approaches towards the generation of architectures for nanostructured materials stresses the broad impact that self-organization may have in nanoscience and nanotechnology, by allowing the potential replacement of tedious and expensive fabrication and addressing procedures by powerful self-fabrication and self-addressing processes.
Starting in the early 1990s, a novel line of research was initiated. It developed from the implementation of a basic feature of supramolecular chemistry, the fact that it is by essence a dynamic chemistry with respect to the constitution of its entities. Indeed, a supramolecular entity may continuously exchange, incorporate/decorporate and rearrange its molecular components, and thus continuously modify its constitution as a consequence of the inherent reversibility of the non-covalent interactions that connect these components. Importing such dynamic features into molecular chemistry requires the intentional introduction of reversible covalent bonds into molecules, so as to confer upon them a plasticity in constitution, characteristic of supramolecular chemistry.
These considerations led to the definition of a general concept, covering molecular as well as supramolecular chemistry, that of constitutional dynamic chemistry (CDC).
Thus, dynamic chemistry, that is usually considered to concern either reaction dynamics or motional dynamics, is extended to the very constitution of chemical entities itself. CDC may have a profound impact on numerous areas of investigation from drug discovery, to materials science and to nanotechnology.
On the molecular/covalent level, CDC covers dynamic combinatorial chemistry (DCC), an approach that, in contrast to classical “static” combinatorial chemistry based on vast collections of prefabricated molecules, implements dynamic libraries whose constituents undergo continuous interconversion by recombination of their building blocks through reversible chemical reactions. Addition of a target molecule to the dynamic set creates a driving force that favours the formation of the best-binding constituent – a self-screening process operating on the basis of molecular recognition between the partners. The application of this methodology to biological systems has allowed the generation of biologically active substances, in particular enzyme inhibitors (carbonic anhydrase, acetylcholine esterase). It is capable, in principle, of accelerating the identification of lead compounds for drug discovery.
In the area of materials science, CDC has been implemented in the development of dynamic polymers, dynamers, reversible polymers of molecular as well as supramolecular nature, such as dynamic polyamides. The potential uses of dynamers resulting from their reversibility have been explored towards applications in areas such as degradable materials and controlled release of active substances through collaborations with companies, leading to a number of patents.
CDC introduces a paradigm shift with respect to constitutionally static chemistry. The latter relies on design for the generation of a target entity, whereas CDC takes advantage of dynamic diversity to allow variation and selection. The implementation of selection in chemistry introduces a fundamental change in outlook. Whereas self-organization by design strives to achieve full control over the output molecular or supramolecular entity by explicit programming, self-organization by selection operates on dynamic constitutional diversity in response to either internal or external factors to achieve adaptation in a darwinistic fashion.
By extending its characteristic features information/programmability, dynamics/reversibility, constitution/structural diversity, supramolecular chemistry is thus impacting molecular chemistry, leading towards the emergence of adaptive and evolutive chemistry.
The research activities of my laboratory at the Collège de France in Paris covered several topics different from but related to those pursued in Strasbourg: – molecular recognition of nucleic acid features by macrocycles containing intercaland groups; – extension of transport processes towards gene transfer through the design of efficient cationic vectors; – implementation of molecular recognition in lipid vesicles, leading to selective aggregation and fusion processes between vesicles doped with complementary recognition groups (recosomes) interacting through hydrogen bonding or metal coordination. Reversible photochemical reactions were used for introducing switching capability into molecular wires and for developing write/read/erase processes for information storage.
In 1998, I set up and directed a research group at the Nanotechnology Institute newly created in the Research Center of Karlsruhe. This allowed to offer to former post-doctoral coworkers the opportunity to develop and to progressively set up independent research activities in nanoscience and nanotechnology.
In the years since 1987, I also had the occasion to engage in activities of general interest. They were as diverse as being Founding Chairman of a new chemical journal “Chemistry, a European Journal”, created in 1995 and truly European as it is now co-owned by 14 European chemical societies. It also gave the starting impetus to a range of European journals (European Journal of Organic Chemistry, European Journal of Inorganic Chemistry, ChemBioChem, ChemPhysChem) that resulted from the termination of national journals of long tradition (such as Berichte der Deutschen Chemischen Gesellschaft, Liebigs Annalen der Chemie, Bulletin de la Société Chimique de France, Gazzetta Chimica Italiana, Recueil des Travaux Chimiques des Pays Bas, Bulletin des Sociétés Chimiques Belges), an all too rare manifestation of European spirit and supranationality bridging historical divides!
A notable activity was the scientific planning of a novel institute ISIS (Institut de Science et d’Ingénierie Supramoléculaires) inaugurated in December 2002, housed in an attractive new building generously financed by the local authorities and provided in equipment and positions thanks to the strong support of the French Ministery of Research. It was possible to assemble a number of high level senior scientists from various countries, promising junior scientists as well as research laboratories from companies in a very stimulating atmosphere.
I have over the years been involved in a number of public and private boards and committees as well as participated in several start-up companies.
Finally, as president of the non-governmental organization IOCD (International Organization for Chemical Sciences in Development), I have tried, with a group of highly dedicated colleagues, to contribute to helping chemists in developing countries.
The scientific work performed now over forty years with about 300 collaborators from over twenty countries has been described in about 800 publications and review papers as well as two books.
| Selected General References |
| “Dynamic combinatorial chemistry and virtual combinatorial libraries“, J.-M. Lehn, Chem. Eur. J., 5, 2455-2463, 1999. |
| “Programmed chemical systems: Multiple subprograms and multiple processing/expression of molecular information“, J.-M. Lehn, Chem. Eur. J., 6, 2097-2102, 2000. |
| “Toward complex matter: Supramolecular chemistry and self-organization“, J.-M. Lehn, Proc. Natl. Acad. Sci. USA, 99, 4763-4768, 2002. |
| “Drug discovery by dynamic combinatorial libraries“, O. Ramström, J.-M. Lehn, Nature Reviews | Drug Discovery, 1, 26-36, 2002. |
| “Supramolecular polymer chemistry – Scope and perspectives“, J.-M. Lehn, Polym. Int. 51, 825-839, 2002. |
| “Self-organization by selection: Generation of a metallosupramolecular grid architecture by selection of components in a dynamic library of ligands“, J.R. Nitschke, J.-M. Lehn, Proc. Natl. Acad. Sci. USA, 100, 11970-11974, 2003. |
| “Supramolecular chemistry: From molecular information toward self-organization and complex matter“, J.-M. Lehn, Rep. Prog. Phys., 67, 249-265, 2004. |
| “Grid-type metal ion architectures: Functional metallosupramolecular arrays“, M. Ruben, J. Rojo, F.J. Romero-Salguero, L.H. Uppadine, J.-M. Lehn, Angew. Chem. Int. Ed., 43, 3644-3662, 2004. |
| “Dynamers: Polyacylhydrazone reversible covalent polymers, component exchange, and constitutional diversity“, W.G. Skene, J.-M. Lehn, Proc. Natl. Acad. Sci. USA, 101, 8270-8275, 2004. |
| “Constitutional dynamic self-sensing in a zinc(II)/polyiminofluorenes system“, N. Giuseppone, J.-M. Lehn, J. Am. Chem. Soc., 126, 11448-11449, 2004. |
| “Dynamers: Dynamic molecular and supramolecular polymers“, J.-M. Lehn, Prog. Polym. Sci., 30, 814-831, 2005. |
This autobiography/biography was written at the time of the award and later published in the book series Les Prix Nobel/ Nobel Lectures/The Nobel Prizes. The information is sometimes updated with an addendum submitted by the Laureate.
Award ceremony speech
Presentation Speech by Professor Inga Fischer-Hjalmars of the Royal Academy of Sciences
Translation from the Swedish text
Your Majesties, Your Royal Highnesses, Ladies and Gentlemen,
The laureates in chemistry of this year have studied the theory of chemical reactions. Chemical reactions is something that fills our daily life. All of us are constantly starting chemical reactions, by turning the key of the car or by cooking at the stove, to say nothing about the endless number of reactions in our own body that follows every breath.
In chemical reactions new compounds are created. It is possible to make designs for their preparation. But these designs are not realiable until the events at the micro level have been understood and the laws have been found that are governing the transformations of the molecules.
A molecule is composed of atoms that are tied together by aid of the electrons. Atomic nuclei and electrons are not at rest but are constantly moving. The paths of the electrons are usually called orbitals. The forms of these orbitals are determining the bonds between the atoms.
In a reaction molecules are impinging against each other. During the collision the electrons are influenced by new atomic nuclei and the orbitals are changed. Some of the bonds are broken and others are created. Afterwards, new molecules have been formed.
What is it then that decides the sequence of events during the collision? One governing factor is the energy. The new molecules are found at a lower energy level than the original ones. Sometimes, the change will take place without difficulty. The reaction complex is simply sliding down an energy slope. But in general some hindrance must be overcome. It is necessary first to go upwards before starting the downhill ride. Then, the problem is to find the lowest passage over the height barrier. Frequently, rather much is known about the starting material and the final product, about the energy valley of the starting point and the valley of the final destination. But about the character of the ridge in between very little has been known. This year’s laureates in chemistry have helped us to foresee the obstacles and to, find the best way to the final goal. The barriers depend on the fact that the electronic orbitals must be transformed. Now, there is a large number of electrons in every molecule, each with its orbital. A drastic simplification of the barrier problem was achieved in the beginning of the 1950’s when Kenichi Fukui discovered that only a few orbitals, those with highest energy, are dominating the frontier of the reaction. He therefore called them frontier orbitals. By use of his theory Fukui found the laws for many groups of organic-chemical reactions. An example: Naphthalene is an important initial material in dye-stuff industry, among others. For a long time the puzzling fact had been known that hydrogen atoms at different positions in the naphthalene molecule are reacting most unequally. The explanation came first through Fukui’s theory.
Many molecules have no stereo-symmetry. Then, the molecule and its mirror image have very different effects, as the right-hand and left-hand are functioning differently. The finger-tips of a right-hand can easily match those of a left-hand, but not those of another right-hand. Usually, chemical reactions will give rise to a mixture of right-and left-molecules, but our bodies are only producing one kind. For the preparation of vitamins and drugs that are to react in the body it is therefore often necessary to use methods giving only right- or only left-molecules. Such methods have been found through an exquisite combination of theory and experiment. The problem to prepare vitamin B12 was attacked among others by the brilliant molecule builder Woodward at Harvard. There was also the theoretician Roald Hoffmann. Together, Hoffmann and Woodward discovered that not only the energy of the orbital but also its symmetry is decisive for the reactivity. Thus, the Woodward-Eschenmoser synthesis of vitamin B12 could be accomplished.
Hoffmann continued to develop the theory of orbital symmetry to an exceedingly practical instrument for synthetic work of widely different character. At the same time, Fukui showed that the frontier orbital theory leads to another powerful method of solving the intricate problems of stereochemistry. In this way, the theoreticians Fukui and Hoffmann have radically changed the conditions for the design of chemical experiments.
Professor Fukui, Professor Hoffmann. Each of you have independently developed important theories of chemical reactivity. The concepts of frontier orbitals and conservation of orbital symmetry have revealed completely new aspects of the interaction between molecules in collision. Through drastic simplifications you have been able to make beautiful generalizations. From your theoretical work new tools have emerged of the greatest importance for the design of chemical experiments. In recognition of your outstanding work the Royal Academy of Sciences has decided to award this year’s Nobel Prize for Chemistry to you.
On behalf of the Royal Academy of Sciences I wish to convey to you our warmest congratulations, and now I ask you to receive your Prizes from the hands of His Majesty the King.