Popular information
English
Swedish

The Nobel Prize in Chemistry 2001
Three scientists share this year’s Nobel Prize in Chemistry: William S. Knowles, previously at Monsanto Company, St. Louis, Missouri, USA; Ryoji Noyori, Nagoya University, Chikusa, Nagoya, Japan and K. Barry Sharpless, The Scripps Research Institute, La Jolla, California, USA. The Royal Swedish Academy of Sciences has awarded the Prize for their development of catalytic asymmetric synthesis. The achievements of these three chemists are of great importance for academic research, for the development of new drugs and materials, and are being used in many industrial syntheses of pharmaceutical products and other biologically active substances. This is a description and background information about the scientists’ award-winning discoveries.
Mirror Image Catalysis
Chiral molecules
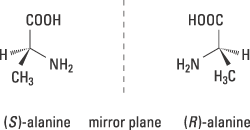
This year’s Nobel Prize in Chemistry concerns the way in which certain chiral molecules can be used to speed up and control important chemical reactions. The word chiral comes from the Greek word cheir, which means hand. Our hands are chiral – our right hand is a mirror image of our left hand – as are most of life’s molecules. If, for example, we study the common amino acid alanine (figure 1), we see that it can occur in two forms: (S)-alanine and (R)-alanine, which are mirror images.
High resolution image (jpeg 45 kB)
Figure 1. Chirality in the amino acid alanine is illustrated with models of its two forms, which are mirror images of each other. They are designated (S) and (R).
However we twist or turn these forms, we cannot get them to overlap each other. Apparently, they do not have the same three-dimensional structure. The reason is that the carbon atom in the centre binds the four different groups H, CH3, NH2 and COOH, which are located at the corners of a tetrahedron. The unbroken bonds to NH2 and COOH indicate that these bonds are in the plane of the paper, whereas the black wedge shaped bond and the broken wedge shaped bond show that they are directed upwards and downwards respectively in relation to the plane of the paper.
It was the Dutch chemist J. H. van ‘t Hoff and the French chemist J. A. Le Bel who, independently of each other in 1874, discovered this tetrahedral arrangement of the groups around the central carbon atom. (van ‘t Hoff received the first Nobel Prize in Chemistry 1901, but for other discoveries.)
Thus the amino acid alanine occurs in two forms, called enantiomers. When alanine is produced in a laboratory under normal conditions, a mixture is obtained, half of which is (S)-alanine and the other (R)-alanine. The synthesis is symmetrical in the sense that it produces equal amounts of both enantiomers.
Asymmetric synthesis, on the other hand, deals with the production of an excess of one of the forms. Why is this so important? Let us go back to nature to find the answer.
Nature is chiral
One may well think that both forms of chiral molecules ought to be equally common in nature, the reactions should be symmetrical. But when we study the molecules of the cells in close-up, it is evident that nature mainly uses one of the two enantiomers. That is why we have – and this applies to all living material, both vegetable and animal – amino acids, and therefore peptides, enzymes and other proteins, only of one of the mirror image forms. Carbohydrates and nucleic acids like DNA and RNA are other examples.
Thus the enzymes in our cells are chiral, as are other receptors that play an important part in cell machinery. This means that they prefer to bind to one of the enantiomers. In other words, the receptors are extremely selective; only one of the enantiomers fits the receptor’s site like a key that fits a lock. (This metaphor comes from another Nobel Laureate in Chemistry, Emil Fischer, who was awarded the Prize in 1902.)
Since the two enantiomers of a chiral molecule often have totally different effects on cells, it is important to be able to produce each of the two forms pure.
Drugs and the smell of lemons
Most drugs consist of chiral molecules. And since a drug must match the molecules it should bind to itself in the cells, it is often only one of the enantiomers that is of interest. In certain cases the other form may even be harmful. This was the case, for example, with the drug thalidomide, which was sold in the 1960s to pregnant women. One of the enantiomers of thalidomide helped against nausea, while the other one could cause foetal damage.
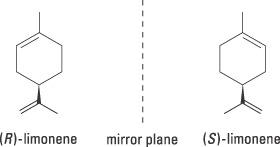
There are other, less dramatic examples of how differently the two enantiomers can affect our cells. Limonene, for example, is chiral, but the two enantiomers can be difficult to distinguish at first glance (figure 2). The receptors in our nose are more sensitive. One form certainly smells of lemons but the other of oranges.
 |
| High resolution image (jpeg 44 kB) |
| Figure 2. (R)-limonene smells of oranges while its enantiomer (S)-limonene smells of lemons |
Catalytic asymmetric synthesis – What is it?
It is very important for industry to be able to produce products as pure as possible. It is also important to be able to manufacture large quantities of a product. For this reason the use of catalysts is very important. A catalyst is a substance that increases the rate of the reaction without being consumed itself.
During the past few decades there has been intensive research into developing methods for producing – synthesising – one of the enantiomers rather than the other. In a synthesis starting molecules (substrate molecules) are used to build new molecules (products) by means of various chemical reactions. It is to researchers in this field that this year’s Nobel Prize in Chemistry has been awarded. The Laureates have developed chiral catalysts for two important classes of reactions in organic chemistry: hydrogenations and oxidations.
Knowles’ pioneer work
In the early sixties it was not known whether catalytic asymmetric hydrogenation was feasible, i.e. would it be possible to catalyse an asymmetric reaction to produce an excess of one of the enantiomers? The breakthrough came in 1968 when William S. Knowles was working at the Monsanto Company, St Louis, USA. He discovered that it was possible to use a transition metal to produce a chiral catalyst that could transfer chirality to a non-chiral substrate and get a chiral product. The reaction was a hydrogenation in which the hydrogen atoms in H2 are added to the carbons in a double bond. A single catalyst molecule can produce millions of molecules of the desired enantiomer.
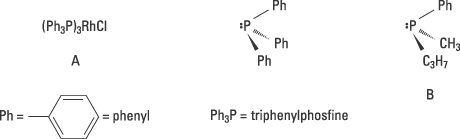
Knowles’ experiments were based on two discoveries that had been made a few years previously. In 1966 Osborn and Wilkinson had published their pioneering synthesis of a soluble transition metal complex, (A in figure 3), that made it possible to catalyse a hydrogenation in solution. Their metal complex was not chiral. At the centre of the complex was the transition metal rhodium which bound four groups, ligands: three triphenylphosphine molecules and one chlorine.
The second discovery on which Knowles’ pioneering work is based on, is Horner’s and Mislow’s syntheses of chiral phosphines, for example the enantiomer B shown in figure 3. Knowles’ hypothesis was that it might be possible to produce a catalyst for asymmetric hydrogenation if the triphenylphosphine groups in Osborn and Wilkinson’s metal complex (A) was replaced by one of the enantiomers of a chiral phosphine.
High resolution image (jpeg 60 kB)
Figure 3. Knowles exchanged the non-chiral phosphine triphenylphosphine in A to the chiral phosphine B and obtained a catalyst for asymmetric hydrogenation.
The phosphine first used by Knowles was not enantiomerally pure, yet it produced a mixture in which there was 15% more of one enantiomer than the other. In other words the enantiomeric excess was 15%.
Although this excess was modest and hardly of any practical use, the result proved that it was in fact possible to achieve catalytic asymmetric hydrogenation. Other scientists (Horner, Kagan, Morrison and Bosnich) reached similar results shortly afterwards and they have all contributed to open the door to a new, exciting and important field for both academic and industrial research.
The first industrial catalytic asymmetric synthesis
Knowles’ aim was to develop an industrial synthesis of the amino acid L-DOPA, which had proved useful in the treatment of Parkinson’s disease – a discovery for which A. Carlsson was awarded last year’s Nobel Prize in Physiology or Medicine. By testing enantiomers of phosphines with a varied structure Knowles and his colleagues quickly succeeded in producing usable catalysts that provided a high enantiomeric excess, that is, principally L-DOPA.
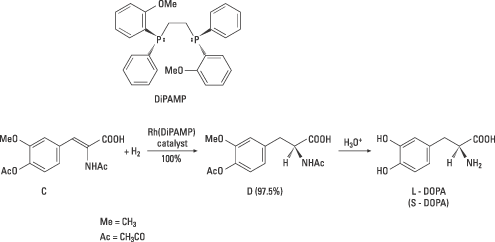
The ligand later used in Monsanto’s industrial synthesis of L-DOPA was the diphosphine ligand DiPAMP. A rhodium complex with this ligand (figure 4) gave a mixture of the enantiomers of DOPA in 100% yield. The product contained of 97.5% L-DOPA. Thus Knowles had in a short time succeeded in applying his own basic research and that of others to create an industrial synthesis of a drug. This was the first catalytic asymmetric synthesis. It has been succeeded by many others.
 |
| High resolution image (jpeg 198 kB) |
| Figure 4. In this industrial synthesis of L-DOPA developed by Knowles and co-workers the compound C was used as the starting material. In the chiral hydrogenation one of the enantiomers of DiPAMP was used. The enantiomer D was 97.5% of the product and after acid hydrolysis of D, L-DOPA was obtained. |
How does a chiral catalyst molecule work?
What part does the catalyst molecule itself actually play in asymmetric hydrogenation? Studies by the inorganic chemist J. Halpern and others have clarified the reaction mechanism. The transition metal, rhodium for example, in figure 4, which binds the chiral diphosphine, has the ability to simultaneously bind both H2 and the substrate. The complex obtained then reacts and H2 is added to the double bond in the substrate. This is the vital hydrogenation stage, when a new chiral complex is formed from which the chiral product is released. Thus from a substrate that is not chiral, chirality has been transferred from the chiral catalyst to the product. This product contains more of one enantiomer than of the other, that is, the synthesis is asymmetric.
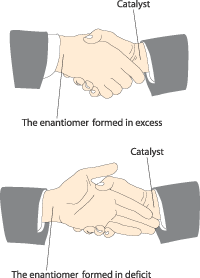
The reason for the enantiomeric excess is to be found in the hydrogenation stage, as the hydrogen can be added in two ways that give the different enantiomers at different rates. These two pathways utilise different transition complexes, which are not mirror images and therefore have different energy. Hydrogenation takes place more rapidly via the complex with the lowest energy, thus producing an excess of one of the enantiomers. This can be compared with the hands in a handshake (figure 5). The hands in a handshake between two right hands match better than a handshake between a right and a left hand.
High resolution image (jpeg 209 kB)
Figure 5. The hands on the right symbolise the catalyst and the hands on the left the products. They match better in the upper picture (the energy is lower) than in the lower picture.
In the development of better asymmetric hydrogenation catalysts it is important to increase the energy difference between the transition complexes in order to obtain, as a consequence, larger enantiomeric excess. This is of vital interest in industrial applications in which the aim is to achieve economy in the process and environmentally acceptable methods, that is, as few waste products as possible. This development has been led by another of this year’s Laureates in chemistry, Ryoji Noyori.
Noyori’s general hydrogenation catalysts
The Japanese scientist Ryoji Noyori has carried out extensive and intensive research and developed better and more general catalysts for hydrogenation. The consequences of his research are of great importance.
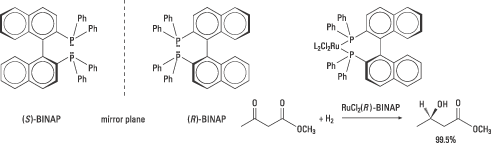
In 1980 Noyori and co-workers published an article on the synthesis of both enantiomers of the diphosphine ligand BINAP (figure 6). These catalyse, in complexes with rhodium, the synthesis of certain amino acids with an enantiomeric excess of up to 100%. The company Takasago International uses BINAP in the industrial synthesis of the chiral aroma substance menthol, since the early 1980s.
 |
| High resolution image (jpeg 229 kB) |
| Figure 6. The two enantiomers of Noyori’s useful BINAP is shown together with an example of a stereoselective ketone reduction where the ester function is left intact. |
But Noyori also saw the need for more general catalysts with broader applications. Exchanging rhodium, Rh(I), for another transition metal, ruthenium, Ru(II), proved, for example, to be successful. The ruthenium(II)-BINAP complex hydrogenates many types of molecules with other functional groups. These reactions give a high enantiomeric excess and high yields and can be scaled up for industrial use. Noyori’s Ru-BINAP is used as a catalyst in the production of (R)-1,2-propandiol for the industrial synthesis of an antibiotic, levofloxacin. Similar reactions are used for the synthesis of other antibiotics. Figure 6 gives an example of a stereoselective ketone reduction.
Noyori’s catalysts have found wide application in the synthesis of fine chemicals, pharmaceutical products and new, advanced materials.
Sharpless’ chirally catalysed oxidations
Alongside the advances in chirally catalysed hydrogenation reactions, Barry Sharpless has developed corresponding chiral catalysts for other important reactions, oxidations. While hydrogenation removes a functional group because the double bond is saturated, oxidation leads to increased functionality. This creates new possibilities for building new complex molecules.
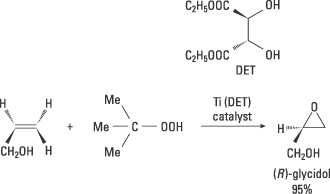
Sharpless realised that there was a great need for catalysts for asymmetric oxidations. He also had ideas as to how these could be achieved. He has made several important discoveries which here are exemplified by his chiral epoxidation. In 1980 he carried out successful experiments that led to a practical method for the catalytic asymmetric oxidation of allylic alcohols to chiral epoxides. This reaction utilised the transition metal titanium (Ti) and chiral ligands and gave high enantiomeric excess. Epoxides are useful intermediary products for various types of synthesis. This method opened up the way for great structural diversity and has had very wide applications in both academic and industrial research. The synthesis of the epoxide (R)-glycidol is shown in figure 7.
High resolution image (jpeg 81 kB)
Figure 7. The allylic alcohol is oxidised to the epoxide (R)-glycidol using the oxidising agent tertiary butylhydroperoxide in the presence of a catalyst. This catalyst is formed in the reaction mixture of titanium tetraisopropoxide and the diethylesther of naturally occurring D-tartaric acid. The metal simultaneously binds the chiral ligand, the hyperperoxide and the substrate, after which the chiral epoxidation takes place.
Glycidol is used in the pharmaceutical industry to produce beta-blockers, which are used as heart medicines. Many scientists have identified Sharpless’ epoxidation as the most important discovery in the field of synthesis during the past few decades.
Consequences and applications
Many of the applications of this year’s Nobel Laureates’ pioneering work have already been discussed. It is especially important to emphasise the great significance of their discoveries and improvements for industry. New drugs are the most important application, but we may also mention the production of flavouring and sweetening agents, and insecticides. This year’s Nobel Prize in Chemistry shows that the step from basic research to industrial application could sometimes be a short one.
All around the world many research groups are busy developing other catalytic asymmetric syntheses that have been inspired by the Laureates’ discoveries. Their discoveries have provided academic research with many important tools, thereby contributing to more rapid advances in research – not only in chemistry but also in materials science, biology and medicine. Their work gives access to new molecules needed to investigate hitherto undiscovered and unexplained phenomena in the molecular world.
The Laureates
William S. Knowles
84 years, born 1917 (US citizen). PhD 1942 at Columbia University. Previously at Monsanto Company, St Louis, USA. Retired since 1986.
Ryoji Noyori
63 years, born 1938 Kobe, Japan (Japanese citizen). PhD 1967 at Kyoto University. Since 1972 Professor of Chemistry at Nagoya University and since 2000 Director of the Research Center for Materials Science, Nagoya University, Nagoya, Japan.
K. Barry Sharpless
60 years, born 1941 Philadelphia, Pennsylvania, USA (US citizen). PhD 1968 at Stanford University. Since 1990 W.M. Keck Professor of Chemistry at the Scripps Research Institute, La Jolla, USA.
Play a game!
Chiral objects, like left and right hands, cannot be superimposed on its mirror image. Find other ‘chiral’ objects in this game.
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 12 laureates' work and discoveries range from proteins' structures and machine learning to fighting for a world free of nuclear weapons.
See them all presented here.
