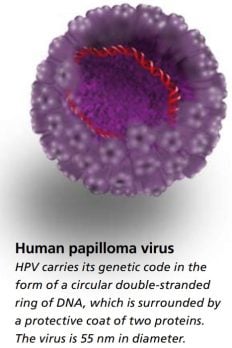
Popular information
Information for the public (pdf)
Populärvetenskaplig information (pdf)

The Nobel Prize in Physiology or Medicine 2008
is awarded with one half to
Harald zur Hausen
for his discovery of “human papilloma viruses causing cervical cancer”
and the other half jointly to
Françoise Barré-Sinoussi and Luc Montagnier
for their discovery of “human immunodeficiency virus”
This year’s Nobel Laureates are being recognized for their discoveries of two different viruses that cause serious illnesses in humans. The discoveries have in common that both have contributed towards reducing human suffering, but here they will be described separately.
Human papilloma virus (HPV): the virus behind cervical cancer is exposed
In 1974 Harald zur Hausen postulated that a chronic infection with as yet unknown types of human papilloma virus, HPV, could give rise to cervical cancer. In doing so, he challenged the hypothesis that prevailed in the research community at that time, namely that cervical cancer was caused by the herpes simplex virus. At a scientific conference, Harald zur Hausen described how he had tried to find herpes viruses in tumors – with no success. He suggested that it might be a good idea to use the same techniques and search for human papilloma viruses, but the other participants at the conference did not receive this suggestion with any enthusiasm.
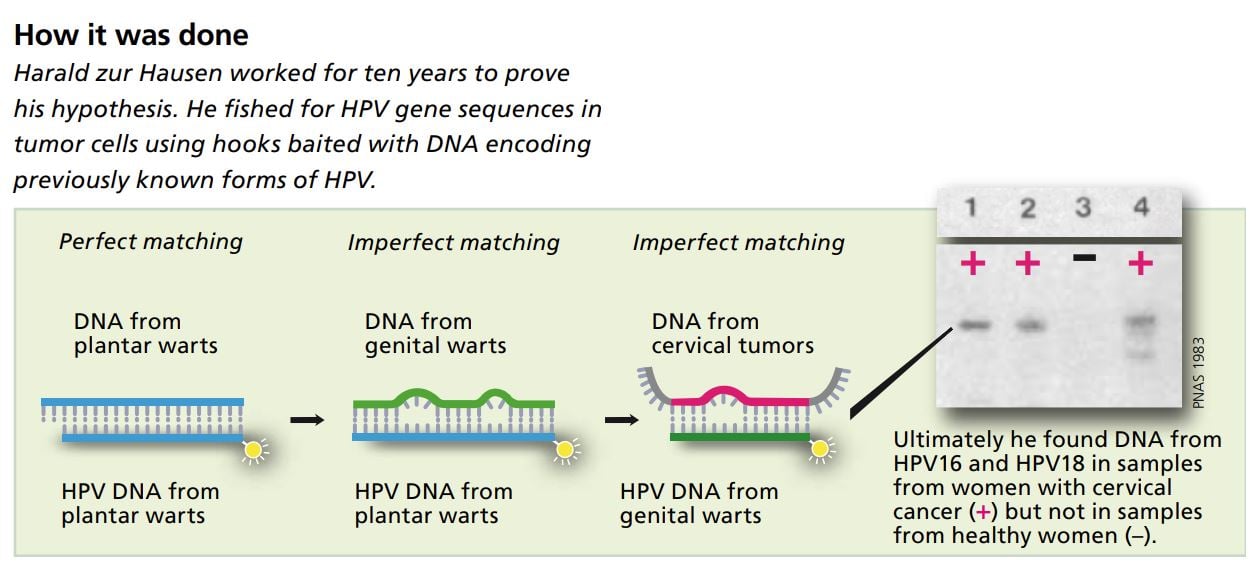
Undiscouraged, zur Hausen embarked on what became several years’ work to test his hypothesis. He assumed that if the tumor cells contained a virus that caused cancer, they would have viral DNA incorporated into their own genes. There the viral DNA could lie dormant for a long time without forming new virus particles. To track down this viral DNA he constructed short, single-stranded DNA segments and used them as “bait” to fish out matching viral DNA from the tumor cells.
This DNA fishing is possible because the bases that make up genetic material can only be paired in a certain way: A with T and C with G. The DNA sequence being used as bait is like the hook side of a hook-and-loop fastener that can only link up with a loop side of HPV carries its genetic code in the the same type.
The technique of using a specific genetic sequence to a protective coat of two proteins.
find a matching sequence is called hybridization.
A patient fisherman
At first, Harald zur Hausen used short, single-stranded segments of viral DNA that he had isolated from cells in plantar warts. It was known that these warts develop after the host is infected with a certain human papilloma virus.
Using these “baited hooks” zur Hausen could easily extract viral DNA from other plantar warts, proving that the technique worked in principle. He was also able to extract viral DNA from ordinary skin warts. But when he used the same bait to fish for unknown HPV DNA in cells from genital warts and cervical tumors, he had no success at first.
He solved this by changing his methods so that the bait was not required to be an exact match for the viral DNA in the cell. By allowing imperfect matching (low stringency hybridization) he was able after ten years’ work to extract segments of DNA from a previously unknown HPV from cervical tumor cells.
In 1983 he described the first cancer-causing HPV type, called HPV16, in an article published in the Proceedings of the National Academy of Sciences. He later identified another new HPV type – HPV18 – in cells from cervical tumors from other patients. He then cloned these two cancer-causing HPV types.
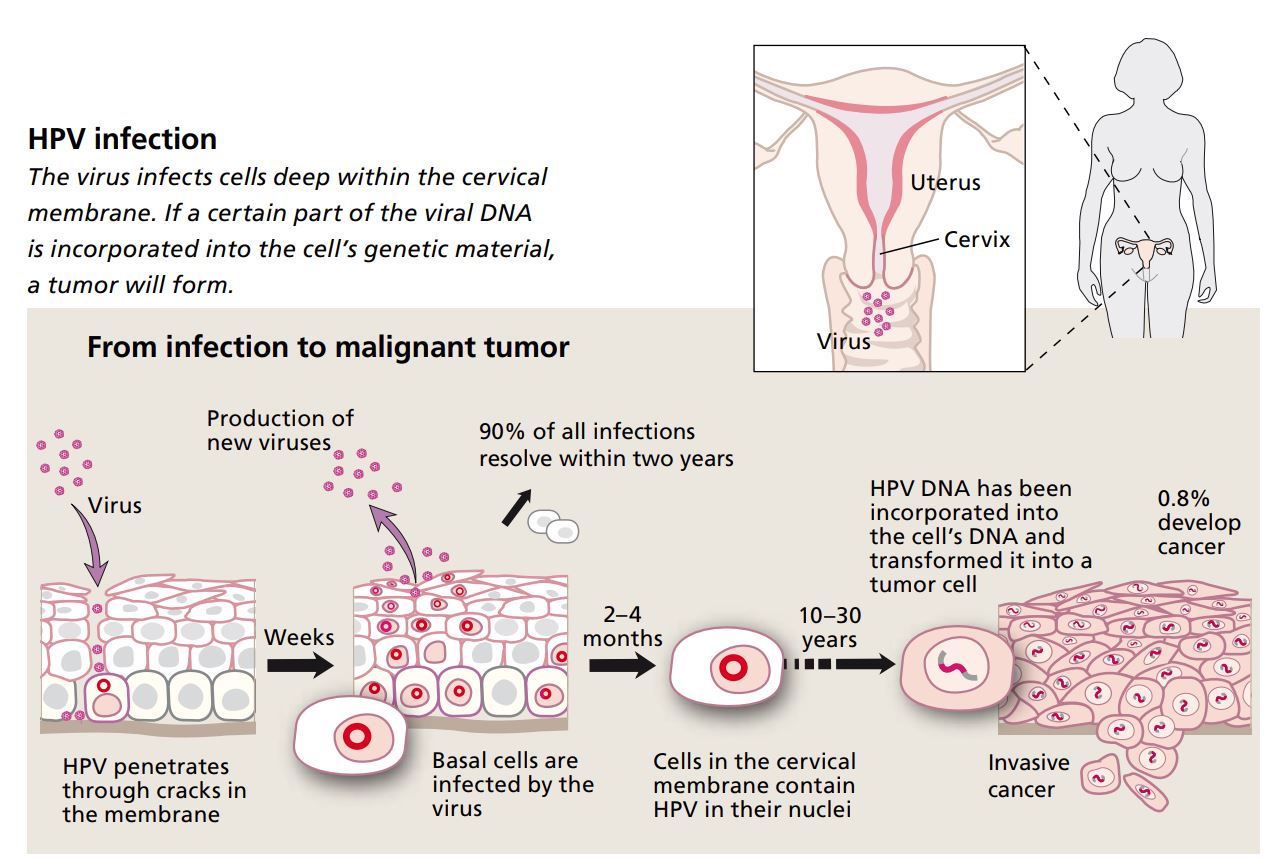
Nine cases out of ten resolve spontaneously
Cervical cancer caused by HPV infection is a sexually transmitted disease. After intercourse, the virus can penetrate to the innermost layer of cells and into the basal cells of the cervical mucous membrane. Basal cells maintain the membrane by dividing and growing outward. The closer the cells get to the surface, the harder and flatter they become. The outermost layer of the membrane consists of dead, keratinized cells that eventually flake off. This means that the membrane provides good protection – as long as it is intact.
When there is an infection, viral DNA penetrates into the nucleus of the basal cells. The virus’s genes stimulate cell replication and the basal cell layer thickens; the outward sign of this is a harmless wart. With time, when the infected cells mature and begin moving upward through the membrane, new virus proteins will be produced. When the cells reach the outermost layer and die, new virus particles are released. The woman is then contagious.
In around one infection out of ten, some viral DNA will be incorporated into the basal cell’s genome. In this case, no new virus particles are produced – only new cells. This is because of the activity of two viral genes, called E6 and E7.
The protein produced by the E7 gene switches off a gene in the basal cell that normally controls cell replication. If the gene is switched off the cell will divide repeatedly. The other gene, E6, encodes a protein that serves to block the function of a protective protein within the cell, called p53. This protective protein normally forces cells that start dividing abnormally to undergo programmed cell death, apoptosis. Dividing abnormally is exactly what a cell starts doing when E7 is active, but if the protective p53 is turned off, the cell can continue to divide. This unchecked cell division means that new genetic changes can occur, and after ten to thirty years, a tumor may form.
Vaccines against HPV save lives
Harald zur Hausen’s work meant that the scientific community had access to HPV16 and HPV18. Many have borne witness to how generously he shared his cloned viruses with colleagues who requested them. He also showed that it is the viral genes E6 and E7 that make it possible for the infection to cause cancer.
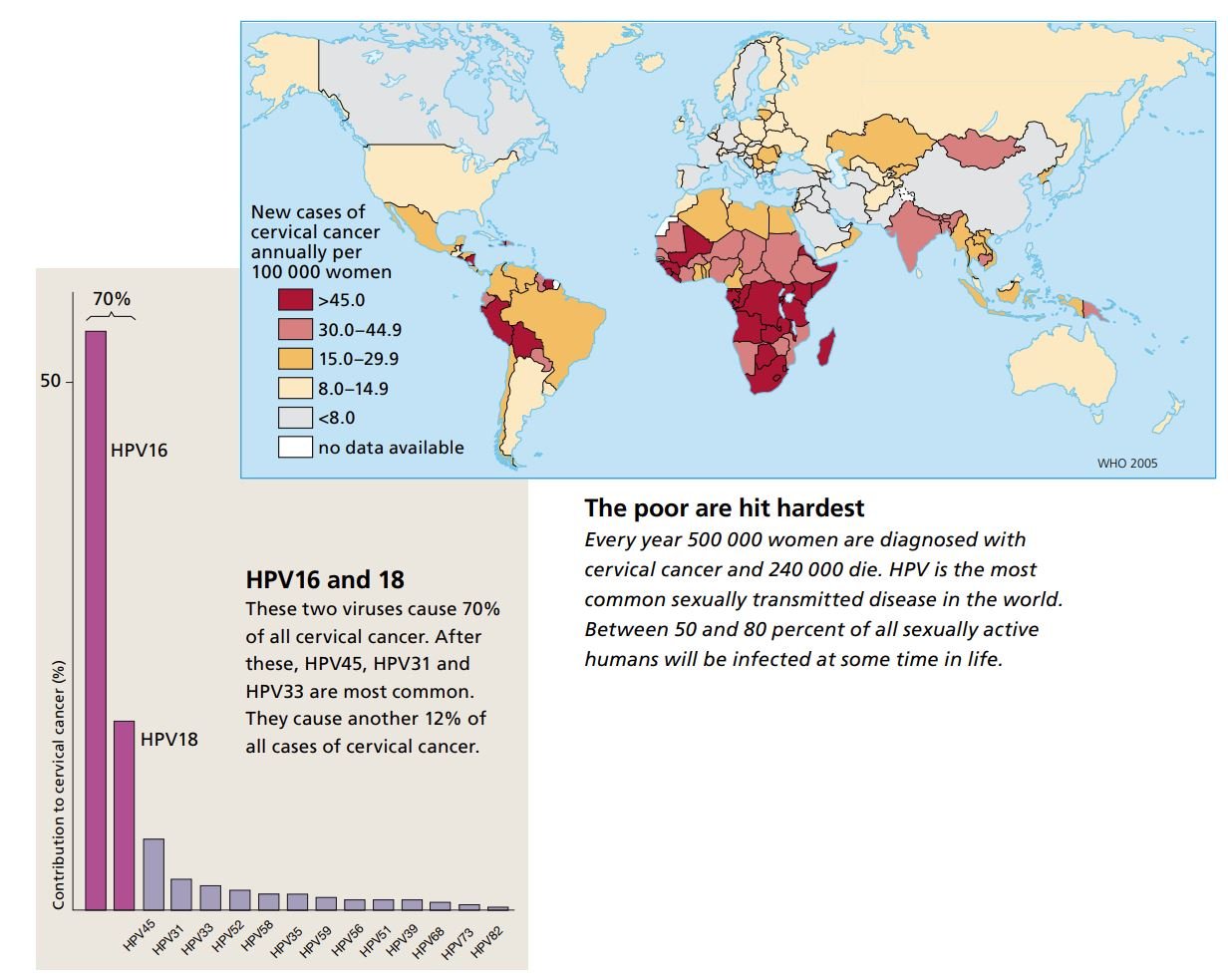
We currently know of about a hundred different types of HPV. Forty of them cause infections in membranes of the genital tract, and fifteen are considered high-risk variants linked to cervical cancer.
It has been estimated that between 50 and 80 percent of the sexually active segment of the world’s population will carry a genital HPV infection at some time during their lives. Every year, about 500 000 women are diagnosed with cervical cancer and 240 000 of these women die. Cervical cancer is one of the most common forms of cancer in women, second only to breast cancer.
Harald zur Hausen and other researchers have shown that in a total of 30 000 women from 25 countries, 70 percent of all malignant tumors contained HPV16 or HPV18. Fortunately we now have vaccines specifically against these viruses. The vaccines have proven to give more than 90 percent protection against the early stages of cancer that these two HPV variants cause.
Human immunodeficiency virus (HIV): the discovery of a deadly new virus
Early in the 1980s we began to hear reports about young men with a strange new spectrum of diseases. They suffered from unusual infections and forms of cancer that were ordinarily seen only in the very old or in people who had been treated with drugs that suppress the immune system.
The first scientific report was published in December 1981 in the New England Journal of Medicine. The article described nineteen young men with severe immune deficiency, twelve of whom had already died. Since most of the cases reported during the first year affected homo- or bisexual men, the condition was first called Gay Related Immunodeficiency. In addition to being transmitted through sexual contacts, infection could also be passed from mother to child and via blood products – even if they had been filtered. This aroused the suspicion that a virus might be involved and an intensive hunt for the causative agent began.
The following year, 1982, it was decided that the serious disease should be called Acquired Immune Deficiency Syndrome, AIDS.
Patients with this disease had so-called opportunistic infections. These are caused by bacteria and microorganisms that our bodies usually have no trouble handling, but that flourish in people whose immune system is weakened. Examples of opportunistic diseases in patients with AIDS include various forms of bacterial pneumonia, severe fungus infections, and a particular type of herpes infection that leads to brownish skin tumors, Kaposi’s sarcoma. Affected individuals also had an increased risk for lymphomas.
Cultured cells from lymph nodes
At Institut Pasteur in Paris, Françoise Barré-Sinoussi and Luc Montagnier were analyzing samples they had received from physicians who had patients with this mysterious immune deficiency.
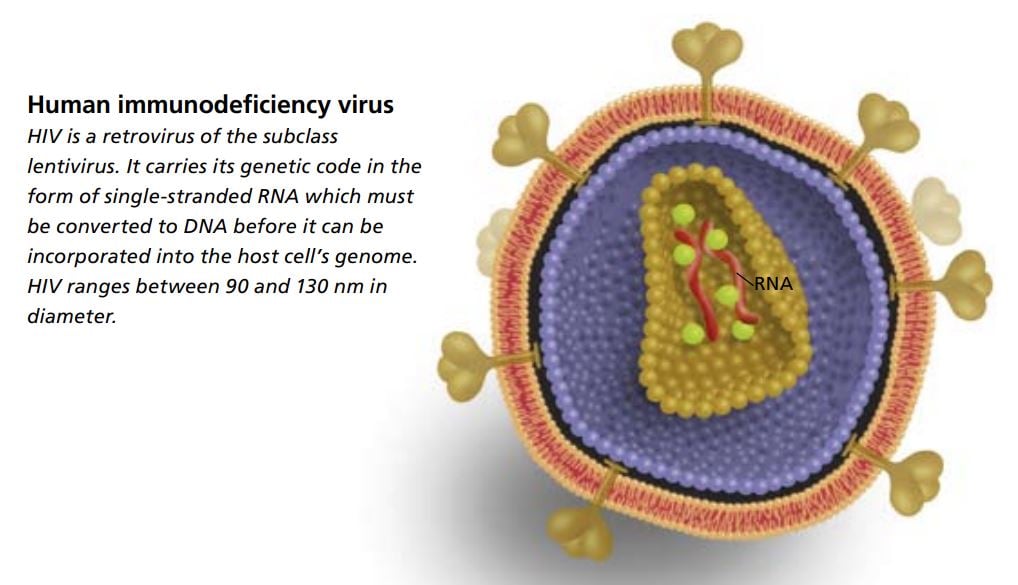
The scientists believed that a retrovirus might be the cause. The term “retrovirus” refers to the fact that these viruses carry their genetic code in the form of RNA. In order to be incorporated into the genome of its host cell, the virus first needs to produce an enzyme called reverse transcriptase, which is able to translate RNA into DNA.
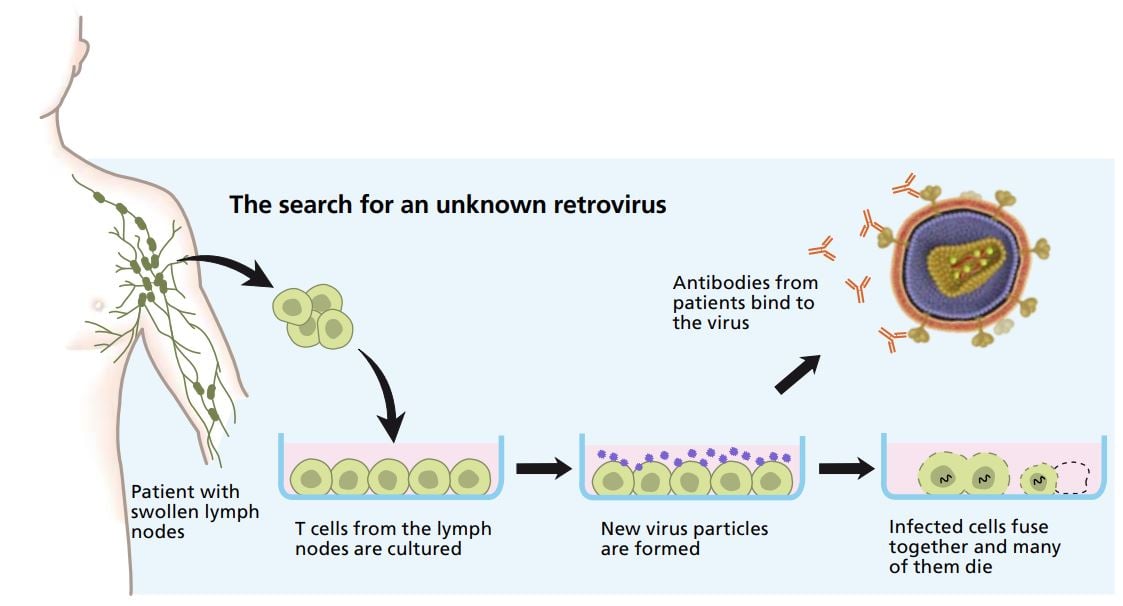
After a meeting with virologists and clinicians in December 1982, Françoise Barré-Sinoussi and Luc Montagnier received lymph nodes from a patient who showed early signs of the disease . With the benefit of hindsight, we can see that this was wise, because lymph nodes contain thousands of times more viruses than blood cells. Cells from lymph nodes were cultured in culture dishes. To get the cells to produce a lot of viruses, they were treated with substances that made them react as if there was an ongoing inflammation.
Enzyme activity reveals a new retrovirus
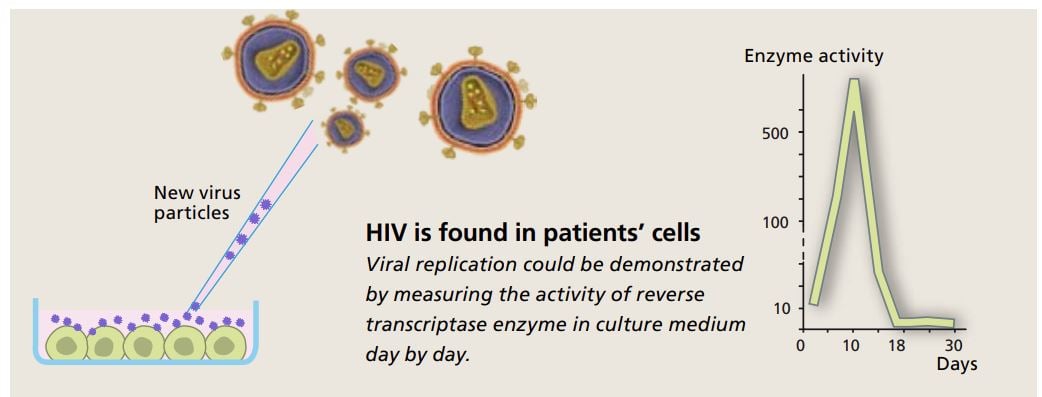
It was possible to harvest viruses from the cell cultures by centrifuging the culture medium. This was done every third day for two weeks. Using the samples containing virus particles Françoise Barré-Sinoussi performed crucial experiments that revealed activity of precisely the type of enzyme retroviruses use, i.e. reverse transcriptase.
When culture medium was centrifuged in a sugar gradient, it was possible to figure out which density showed the highest enzyme activity – and contained the largest number of viruses. This density correlated well with what had previously been reported for retroviruses.
To obtain further confirmation, she examined the enzyme activity in greater detail. This activity cannot be examined directly, but one can use an indirect method and test which metal ions the enzyme requires in order to transcribe RNA. There are different types of reverse transcriptase, for instance inside mitochondria, the cell’s energy factories. Mitochondrial reverse transcriptase requires manganese ions, but the enzyme she had found required magnesium ions. This clearly showed that it came from a retrovirus.
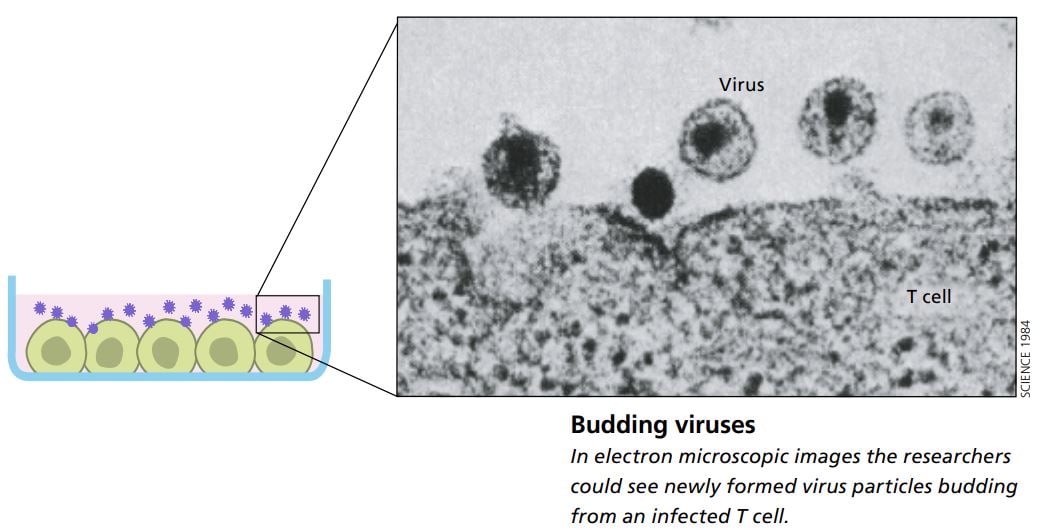
Viral budding
Françoise Barré-Sinoussi, Luc Montagnier and their colleagues were also able to show that the cells the viruses infected were a certain type of leukocytes called T helper cells. When infected, some of these white blood cells fused together and died, which explained why the infected patients had so few leukocytes of this type.
Even during their very first experiments, the researchers had noted that the cells taken from lymph nodes died. This indicated that their virus was of a type that differed from previously identified lymphoproliferative tumor-forming retroviruses. To help the cell cultures survive, they added fresh cells from healthy humans to the culture dishes (from samples they received from the blood bank at Institut Pasteur). These initially healthy cells got infected with the virus and became contagious themselves.
With the help of an electron microscope the scientists were able to study how newly formed virus particles budded off from the infected cells. The virus’s appearance, its morphology, showed that it belonged to a subgroup of retroviruses; it was a lentivirus.
They published their findings in the journal Science in May 1983. At the time they called their virus Lymphadenopathy Associated Virus (LAV).
HIV gets its name
The word “lenti” is Latin for slow. Scientists had learned from studies on animals that lentiviruses give rise to diseases that develop slowly. The actual production of virus particles, on the other hand, is rapid; huge numbers of viruses are produced in just a short time, killing the infected cells. The newly isolated virus was the first lentivirus ever found that could infect humans.
In 1985 the first commercial blood test to detect this virus became available. An international virus taxonomy consortium decided then that the virus should bear the name it is now known by: HIV-1, for Human Immunodeficiency Virus type 1.
Four years after the identification of HIV the World Health Organization, WHO, launched a program to combat HIV internationally. But it was an uphill battle. One year later it was clear that the epidemic had spread all around the globe.
A French discovery
Many research groups published reports in 1984 and 1985 confirming that HIV causes AIDS. This year’s prize is being awarded for the actual discovery of the virus, which happened in Paris.
Since 1981, when the first few cases were noted in the United States, sixty million people around the world have been infected with HIV, and a total of twenty-five million have died of AIDS. The disease is now most prevalent in sub-Saharan Africa. Heterosexual transmission dominates and young women are particularly vulnerable.
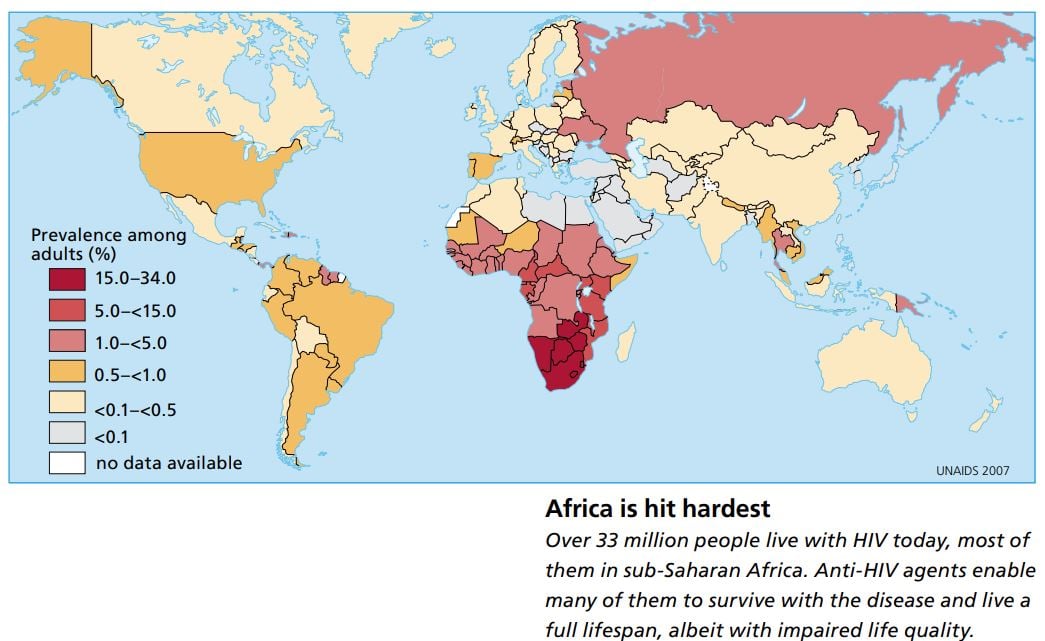
Once we were able to recognize the virus and the antibodies the immune system produces to try to combat infection, it became possible to diagnose the disease and test blood products. Testing is necessary to eliminate infected blood and prevent the disease from spreading to hemophiliacs and others who rely on blood products.
When the virus had been cloned it was also possible to identify proteins that were specific to this virus. That made it possible to develop antiviral drugs; access to such drugs has among other things enabled us to prevent children from being infected by their mothers at birth.
In 2006, scientists from the United States demonstrated conclusively that the virus originated from chimpanzees: they discovered a simian variant of HIV in wild apes. Before then the virus had only been found in apes in captivity. It is likely that the virus passed over into humans with blood from apes that were hunted. Calculations of how many times the virus has mutated suggest that the first ape-to-human transmission took place in West Africa as early as around 1900.
No cure yet
As soon as it was clear what virus was causing this stealthy disease, hope awoke that we would soon be able to develop an effective vaccine. But it turned out to be too complicated for that. This is partly because HIV is highly changeable and swiftly alters form, but also because the virus underhandedly infects and kills precisely the cells in the immune system that are critical in triggering the immune reactions that ordinarily protect us.
As yet there is no complete cure, but if treated with combinations of antiviral drugs, patients can survive for a near-normal lifespan, though their life quality is impaired.
More and more people are gaining access to these drugs, which are also becoming less expensive. A daily dose now costs less than 2 USD. But still the antiviral drugs do not reach all those who need them, partly because of distribution problems, partly because of lack of knowledge about how the drugs should be taken. Of the world’s 33 million infected people, six to nine million are considered to need treatment, and three million also have access to it (two million of them in developing countries).
The fact that we no longer see severely ill, emaciated people with AIDS in the western world has unfortunately led to the misconception that the disease can be cured. This in turn means that many now do not bother to protect themselves when they have casual sexual contacts. This is risky because HIV much more easily infects people who already carry some other infection.
The discoveries have reduced human suffering
This year’s prize recognizes the scientists who have discovered two of humankind’s worst killers. The cancer-causing HPV types and HIV are both spread mainly through sexual contacts. They are found all over the world, but the burden of disease is worst in poor countries. The identification of these viruses has put the research community in a better position to develop methods both to halt the spread of infection and to reduce mortality.
Harald zur Hausen’s discovery of the tumor-causing HPV types HPV16 and HPV18 enabled development of effective vaccines against these two forms of HPV. It is reasonable to hope that today’s or tomorrow’s vaccines will prevent countless women from getting cervical cancer.
That Françoise Barré-Sinoussi and Luc Montagnier identified HIV so quickly has been a great advantage, because their discovery meant that many never got the infection. Detailed studies of how the virus infects host cells and how it is replicated have made it possible to develop drugs that markedly increase both the lifespan and the life quality of people with this infection.
Previous Nobel Prizes in this research field
This year’s award touches on aspects of several previous Nobel Prizes awarded for research on how the genome is organized, molecular biological techniques that make it possible to produce specific DNA sequences, and how the immune system and viruses function.
1908: Ilya Ilyich Mechnikov and Paul Ehrlich “in recognition of their work on immunity”.
1954: John F. Enders, Thomas H. Weller and Frederick C. Robbins “for their discovery of the ability of poliomyelitis viruses to grow in cultures of various types of tissue”.
1965: François Jacob, André Lwoff and Jacques Monod “for their discoveries concerning genetic control of enzyme and virus synthesis”.
1966: Peyton Rous “for his discovery of tumour-inducing viruses”.
1975: David Baltimore, Renato Dulbecco and Howard Martin Temin “for their discoveries concerning the interaction between tumour viruses and the genetic material of the cell”.
1978: Werner Arber, Daniel Nathans and Hamilton O. Smith “for the discovery of restriction enzymes and their application to problems of molecular genetics”.
1989: J. Michael Bishop and Harold E. Varmus “for their discovery of the cellular origin of retroviral oncogenes”.
1996: Peter C. Doherty and Rolf M. Zinkernagel “for their discoveries concerning the specificity of the cell mediated immune defence”.
In addition to these Nobel Prizes in Physiology or Medicine, research in this field has been awarded a Nobel Prize in Chemistry.
1980: Paul Berg “for his fundamental studies of the biochemistry of nucleic acids, with particular regard to recombinant-DNA” and Walter Gilbert and Frederick Sanger “for their contributions concerning the determination of base sequences in nucleic acids”.
The editorial committee for this year’s popular presentation of the Nobel Prize in Physiology or Medicine included the following, who are professors at Karolinska Institutet and members of the Nobel Assembly: Jan Andersson, Bertil Fredholm, Hans Jörnvall, Klas Kärre and Björn Vennström
Text: Lotta Fredholm, science journalist
Translation: Janet Holmén, editor
Illustrations: Bengt Gullbing and Annika Röhl
Virus image accuracy check: professor Henrik Garoff, Karolinska Institutet
© 2008 The Nobel Committee for Physiology or Medicine 2008, Karolinska Institutet, 171 77 Stockholm
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 12 laureates' work and discoveries range from proteins' structures and machine learning to fighting for a world free of nuclear weapons.
See them all presented here.
