Popular information
Information for the public:
Metathesis – a change-your-partners dance (pdf)
Populärvetenskaplig information:
Metates – en dans med partnerbyte (pdf)

The Nobel Prize in Chemistry 2005
This year’s Nobel Prize in Chemistry is to be shared by three scientists: Frenchman Yves Chauvin and Americans Robert H. Grubbs and Richard R. Schrock. The Royal Swedish Academy of Sciences citation runs “for the development of the metathesis method in organic synthesis”. The Laureates’ contributions have already assumed major significance in the chemicals industry, opening up new opportunities for synthesising molecules that will streamline the development and industrial production of pharmaceuticals, plastics and other materials. Production will become cheaper and more environmentally friendly.
Metathesis – a change-your-partners dance
What is metathesis?
In chemical reactions the bonds between different atoms are broken and new bonds formed. The reaction in the focus of this year’s Nobel Prize in Chemistry is metathesis, a word meaning ‘change places’. In olefin metathesis (olefin is another name for alkene, a carbon chain with double bonding) the double-bonding atom groups will change places with one another (Fig. 1).
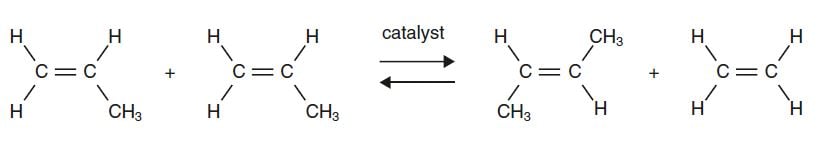
Figure 1. Two propene molecules undergo olefin metathesis with the help of a catalyst, producing two new alkenes, butene and ethene (ethylene).
© The Royal Swedish Academy of Sciences
In this metathesis reaction one of the propene molecules exchanges its CH2 group for the CH3CH group in the other propene molecule. The result is butene and ethene. A catalyst, which is not consumed in the reaction, is required for the reaction to occur.
It has long been possible to produce new substances in this way – without understanding the catalyst’s role in the reaction. Yves Chauvin’s reaction mechanism represents a great step forwards since it showed how the catalyst functions. Here, researchers were given a new challenge to grapple with: to construct new efficient catalysts. Here Robert H. Grubbs’ and Richard R. Schrock’s fundamental research enters the picture. Thanks to their contributions we have the catalysts that are so useful today.
Organic compounds – an enormous diversity
The element carbon has a fantastic ability to form strong bonds to other carbon atoms, and to other elements such as hydrogen, oxygen, chlorine and sulphur. A carbon atom can bind other atoms with single, double or triple bonds and form chains, branched structures and rings of different forms and sizes. This area of chemistry is called organic chemistry, since all life on Earth is based on the versatility of carbon.
So far, only an extremely small proportion of the enormous quantity of different organic molecules has been investigated. Even so, we have already gained new pharmaceuticals, materials, coatings etc., undreamed-of a few years ago.
Organic synthesis
Synthesis involves producing different substances by making substances react in certain ways: new molecules are built with the help of other molecules. Many industries use organic synthesis: pharmaceutical and biotechnical industries as well as in the cellulose and fine chemicals. In Fig. 2 the synthesis of a substance, A, (needed in cancer research) from a molecule, B, is shown. B, in turn, is synthesised from other molecules. Substance B contains a long chain of carbon atoms in which a carbon has been replaced by an oxygen. In the synthesis of A the long chain has become a large ring, which is required for the anti-cancer activity.
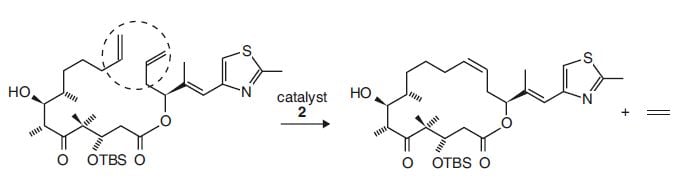
Figure 2. Synthesis with one of Grubbs’ catalysts. Metathesis is used to produce the large ring in A from the long chain in B. Substance A is used in cancer research where the ring is needed to give anti-cancer activity.
© The Royal Swedish Academy of Sciences
How metathesis was discovered
Metathesis was discovered as early as in the 1950s. Just like many other discoveries in organic chemistry, it all began in industry. A number of patents described the catalysation of olefin polymerisation. Among these documents was a report by H. S. Eleuterio at DuPont, USA, from 1957 describing the formation of carbon chains with double bonds, i.e. unsaturated polymers (olefins). However, earlier attempts to polymerize the olefin ethene to polyethene had produced saturated polymers (no double bonds).
This surprising discovery was to have far-reaching consequences. In the same year another patent showed that propene could be converted into butene and ethene when treated with a mixture of triisobutyl aluminium and molybdenum oxide on aluminium oxide. This reaction is shown in Fig. 1 – the Phillips triolefin process. Both discoveries have been successfully used in industry.
The connection between these two discoveries were made much later, by N. Calderon at the Goodyear Tire and Rubber Company, USA. Calderon showed that the same type of reaction took place in both processes. He termed the reaction olefin metathesis. However, the appearance of the catalyst at molecular level, and how it worked, were still unknown. Thus the start of the exciting hunt for other catalysts for the reaction was pure hit-and-miss – fumbling in the dark.
The Chauvin mechanism
More and more chemists started to realise that metathesis could offer great potential in organic synthesis. But no-one suspected how great it was to become. Though many researchers put forward proposals as to how metathesis could take place, the breakthrough came in 1970 with a publication by Yves Chauvin. He and his student Jean-Louis Hérrison proposed that the catalyst was a metal carbene (a compound in which the metal is bound to the carbon with a double bond). In later literature metal carbene came to be termed metal alkylide. Other metal carbenes had been discovered some years earlier by E. O. Fischer (Nobel Prize in Chemistry 1973). Chauvin also presented an entirely new mechanism for how the metal compound functions as a catalyst in the reaction. His new experimental results tallied with this new mechanism and could not be explained by any previously proposed mechanism. In Fig. 3a a metal methyl acts as catalyst for the exchange of alkylidenes between two different alkenes and the result is two entirely new alkenes (the square brackets round the M show that the metal, apart from being bound with a double bond to methylene, is also bound to two other groups).
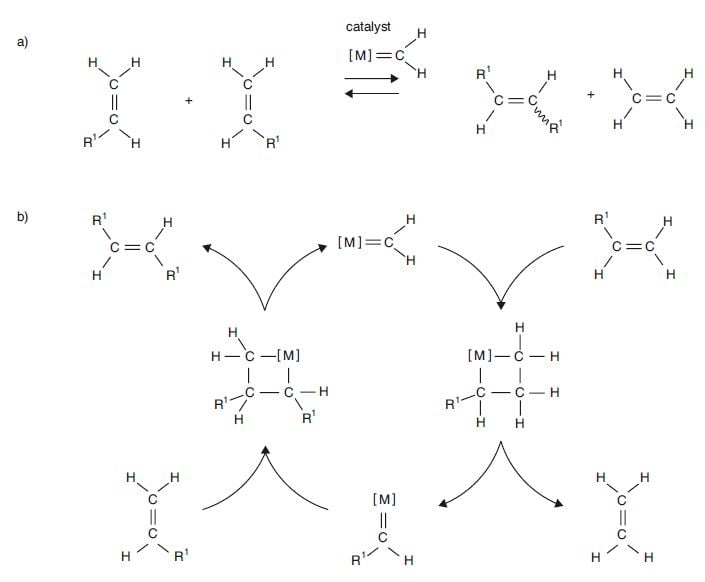
Figure 3. (a) Metathesis of alkenes catalysed by a metal alkylide. The products are two new alkenes – ethene and the alkene with two R1 groups – one on each carbon in the double bond. The wavy bond shows that the R1 groups can be located on the same side of the double bond or on different sides. (b) Chauvin’s mechanism for olefin metathesis. In the catalytic cycle on the way to the products rings with four atoms are formed – three carbons and one metal.
© The Royal Swedish Academy of Sciences
Fig. 3b shows the mechanism. In the first stage of the reaction metal methylene combines with one alkene to form a ring of four atoms. The ring consists of the metal atom and three carbon atoms bound to one another with single bonds. In the next step two of the single bonds are broken and a new alkene (ethene) and a new metal alkylidene is obtained. In the third step of the reaction this new metal alkylidene unites with one of the original alkenes to form a new metallocyclobutane. In the last stage of the catalytic cycle this transition molecule is broken apart and gives a metathesis product and the re-formation of the metal methylene molecule. It is now ready to act as a catalyst in another metathesis reaction. The end result of the reaction cycle is that the two substrate molecules have exchanged alkylidene groups with each other; they have undergone metathesis (Fig. 3a). Chauvin’s mechanism explained at one stroke all earlier outcomes of olefin metathesis. His mechanism received strong support form experimental investigations by Robert H. Grubbs, Thomas J. Katz and Richard R. Schrock, and is now generally accepted.
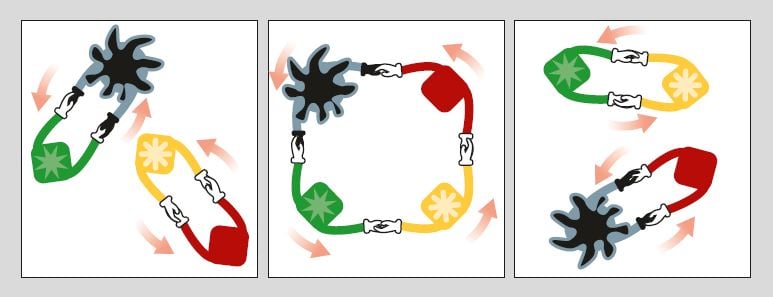
Figure 4. Chauvins’ mechanism described above can be viewed as a dance in which the “catalyst pair” and the “alkene pair” dance round and change partners with one another. The metal and its partner hold hands with both hands and when they meet the “alkene pair” (a dancing pair consisting of two alkylides) the two pairs unite in a ring dance. After a while they let go of each other’s hands, leave their old partners and dance on with their new ones. The new “catalyst pair” is now ready to catch another dancing “alkene pair” for a new ring dance or, in other words, to continue acting as a catalyst in metathesis.
© The Royal Swedish Academy of Sciences
Developing catalyst molecules
More and more chemists now started to realise that metathesis could assume great importance for organic synthesis if reliable and effective catalysts could be found. Earlier, undefined catalysts were used, sensitive to air and moisture and also relatively short-lived. The requirement was stable and well-defined catalysts with reactivity that could be adjusted depending on the purpose. In addition they had to be selective – only react with double bonds and leave other parts of the molecules intact. Chauvin’s results showed how efficient metal alkylidene catalysts could be constructed. The problem was that none of the known well-defined metal alkylidenes acted as catalysts in olefin metathesis. A number of chemists made major contributions to the development of metathesis catalysts and their applications; but the crucial progress in this area was made by Robert H. Grubbs and Richard R. Schrock.
Schrock’s first practicable catalysts
Richard Schrock started research on new alkylidene complexes in the early 1970s. But what metal was the best to use in order to achieve the most effective catalyst? He tried catalysts containing different metals such as tantalum, tungsten and molybdenum. He gradually developed an understanding of what metals could be used in the catalysts and how they functioned. For Schrock, molybdenum and tungsten soon appeared to be the most suitable metals. Some catalysts were produced with those metals, but there was still uncertainty as to what groups would bind to the metal to give stable yet active alkylidene complexes. A breakthrough came in 1990 when Schrock and co-workers reported the construction of a group of very active, well-defined molybdenum catalysts (Fig. 5).
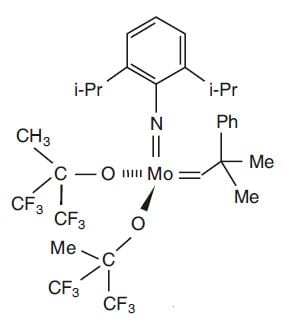
Figure 5. One of Schrock’s molybdenum catalysts. High reactivity has been achieved with the specially-selected groups bound to the metal atom (i-Pr=iso-propyl and Ph=Phenyl).
© The Royal Swedish Academy of Sciences
With this discovery chemists began to realise that olefin metathesis can be used for general purposes in organic synthesis. Metathesis gained increasing attention among researchers active in synthetic chemistry. It turned out that metathesis can replace a number of traditional synthesis methods. At the same time it permits entirely new approaches to the synthesis of organic molecules. Molybdenum catalysts, such as 5, are sensitive to e.g. oxygen and moisture but, with the right treatment, are very powerful tools in organic synthesis.
General catalysts developed by Grubbs
Yet another breakthrough in the development of metathesis catalysts came in 1992 when Robert Grubbs and his co-workers published their discovery of a catalyst with the metal ruthenium. It was stable in air and demonstrated higher selectivity but lower reactivity than Richard R. Schrock’s catalysts. The new catalyst also had the ability to initiate metathesis in the presence of alcohols, water and carboxyl acids (compare with Fig. 2). Grubbs afterwards improved his catalysts and in Fig. 6, one of his effective metathesis catalysts that are easy to synthesise are shown.
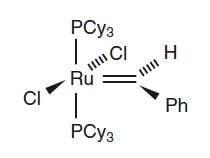
Figure 6. Ruthenium catalyst developed by Grubbs (Ph=phenyl and Cy=cyklohexyl).
© The Royal Swedish Academy of Sciences
Grubbs’ catalysts have become the first well-defined catalysts for general metathesis applications in ordinary laboratories. Catalyst 2 in fig. 6 is generally named Grubbs’ catalyst and has become a standard with which all new catalysts are compared. The general applicability of Grubbs’ catalyst has given rise to future prospects of the possibilities of organic synthesis. Grubbs bases his catalyst design on detailed mechanical studies. He has continued development of ruthenium-based metathesis catalysts into yet more powerful tools for synthesis, including that of polymers with special properties.
Applications and consequences
The synthesis methods developed by the Laureate’s have rapidly become common tools in academic research. An intense development is also carried out in designing industrial processes for production of new substances. With catalytic metathesis the synthesis routes are shorter, giving more product and fewer restproducts. This leads to cleaner and more environmentallyfriendly production. The reaction has opened up greater opportunities to exploit the diversity of organic molecules. Many researchers, besides the Laureates, have made important contributions and are continuing the development of new metathesis catalysts characterised to solve specific problems. Examples are the synthesis of complicated natural products and similar compounds.
Metathesis has great commercial potential in the pharmaceuticals industry, the biotechnical industry and in foodstuffs production. The new metathesis catalysts are also widely applicable in polymer synthesis, although, to date, most successful polymer materials have been manufactured with traditional methods. The latest research in polymer synthesis shows that certain metathesis catalysts have a bright future in the tailoring of polymers with special characteristics.
Considering the relatively short time Schrock’s and Grubbs’ catalysts have been available it is remarkable to note the breadth of applications they have found. These include the synthesis of insect pheromones (Advanced information on the Nobel Prize in Chemistry 2005, see further reading page 7), herbicides, additives for polymers and fuels, polymers with special properties and various substances of interest in pharmaceuticals development. The development of molecules that attack various bodily illnesses merits further mention, since researchers are now devoting themselves to the creation of pharmaceuticals candidates for treating such widely differing conditions as bacterial infections, hepatitis C, cancer, Alzheimer’s disease, Down’s syndrome, osteoporosis, arthritis, inflammation, fibrosis, HIV/AIDS, migraine, etc. Metathesis is thus an important weapon in the hunt for new pharmaceuticals for treating many of the world’s major diseases.
LINKS AND FURTHER READING
At the website of the Nobel Prizes, www.nobelprize.org, one can find more information on this year’s Prizes, e.g. the press conference as web-TV. There is also a scientific article, for the more advanced reader.
Further reading
Advanced information on the Nobel Prize in Chemistry 2005. The Royal Swedish Academy of Sciences
Chemical & Engineering News: Cover story – Olefin Metathesis, December 23, 2002 Volume 80, Number 51, ISSN 0009-2347, Big-deal reaction pp. 29-33. The early days pp. 34-38.
Schuster, M., Blechert, S. (2001) Die Olefinmetathese – neue Katalysatoren vergrößern das Anwendungspotential. Chemie in unserer Zeit, 1, 24
The Age of the Molecule, N. Hall., Royal Society of Chemistry, London, 1999. Classics in Total Synthesis II, K. C. Nicolaou and S. A. Snyder, VHC, Weinheim, 2003.
Links
www.kva.se/swe/awards/nobel/nobelprizes/press/chemread05.asp
http://pubs.acs.org/cen/coverstory/8051/print/8051olefin2.html
http://web.mit.edu/newsoffice/1996/schrock.html
www.its.caltech.edu/~dmacgrp/grpmtgs/2000/WSJ-RCM.pdf
http://pubs.acs.org/cen/coverstory/8051/8051olefin.html
www.rsc.org/delivery/_ArticleLinking/DisplayArticleForFree.cfm?doi=b412198h
THE LAUREATES
YVES CHAUVIN
Institut Français du Pétrole, 1&4, avenue de Bois-Préau 92852 Rueil-Malmaison, Frankrike
French Citizen. Born 1930 (74 years). Directeur de Research Honoreur, Institut du Pétrole, Rueil-Malmaison, France.
ROBERT H. GRUBBS
The Division of Chemistry and Chemical Engineering, California Institute of Technology (Caltech)
363 Crellin 164-30, Pasadena, CA 91125, USA
www.cce.caltech.edu/faculty/grubbs/
US citizen. Born 1942 (63 years) in Calvert City, KY, USA. PhD in chemistry in 1968 from Columbia University, New York, NY, USA.
Victor and Elisabeth Atkins Professor of Chemistry at California Institute of Technology (Caltech), Pasadena, CA, USA.
RICHARD R. SCHROCK
Department of Chemistry, Massachusetts Institute of Technology (MIT)
77 Massachusetts Ave. 6-331, Cambridge, MA 02139 USA
http://web.mit.edu/chemistry/www/faculty/schrock.html
US citizen. Born 1945 (60 years) in Berne, IN, USA. PhD in chemistry in 1971 from Harvard University, Cambridge, MA, USA. Frederick G. Keyes Professor of Chemistry at Massachusetts Institute of Technology (MIT), Cambridge, MA, USA.
© The Royal Swedish Academy of Sciences
Nobel Prizes and laureates
Six prizes were awarded for achievements that have conferred the greatest benefit to humankind. The 12 laureates' work and discoveries range from proteins' structures and machine learning to fighting for a world free of nuclear weapons.
See them all presented here.
